Long SARS-CoV-2 with Hypoxic Leukoencephalopathy, Cardiocirculatory Arrest and Severe Interstitial Pneumonia: The Role of Timely Nuclear Magnetic Resonance for the Early Diagnosis and Regression of this Dramatic Complication
A B S T R A C T
In December 2019, a devastated novel coronavirus, Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2), was identified as the causative agent of the acute atypical cluster of pneumonia cases in the city of Wuhan, China [1]. In February 2020, the World Health Organization (WHO) named the disease COVID-19. Currently, the SARS-CoV-2 pandemic is of a scale not seen since the 1918 influenza pandemic and has already infected more than 156 million people worldwide and resulted in 3.2 million deaths, and cancer is a major risk factor for death associated with COVID-19. Although the predominant clinical presentation is with respiratory disease, Human coronaviruses exhibit neuroinvasive potential, and the systemic disorders that are the hallmark of COVID-19, such as hypoxia, inflammation, and acquired thrombophilia, may impose a high risk of CNS complications [2]. However, it is not clear which of these complications are consequences of direct neurological injury of SARS-CoV-2 or events secondary to the systemic dysfunctional state. New information about the neurological entities arising in patients with COVID-19 is needed to delineate better the clinical implications of this disease. We report a case of a patient with Hypoxic Leukoencephalopathy after Cardiocirculatory Arrest in patient SARS-CoV-2 and Severe Interstitial Pneumonia.
Keywords
Hypoxic leukoencephalopathy in SARS-CoV-2 patient, cardiocirculatory arrest
Case Presentation
Our Italian patient 68-year-old, men, developed fever up to 38.8℃, asthenia, myalgia, dyspnea, cough, seizure, altered mental state, headache, visual disturbances on 16 September 2020. In the Hospital he was admitted immediately after computed tomography (CT) imaging of his chest showed multiple and bilateral ground-glass opacities located in both subpleural and apico-basal spaces (especially on the left) and extensive left spontaneous pneumothorax with subtotal lung collapse. Nasopharyngeal swab specimens were collected to detect severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) nucleic acid. The swab specimens were tested by real-time reverse transcriptase–polymerase chain reaction; a positive result was received 2 days later on 18 September 2020. Our patient was diagnosed with COVID-19 and not recommended treatment with Remdesivir for nephrotoxicity. He received 400 mg of moxifloxacin I.V daily for 3 days; O2 Therapy; methylprednisolone three i.v. boluses of 200mg; Tocilizumab was given in a single i.v. 400-mg dose; prophylactic enoxaparin was prescribed (he no presented thrombotic evets). The patient had a history of Arterial Hypertension compliant with medications, obesity, chronic renal impairment secondary to hypertensive nephropathy [baseline eGFR 22 (normal >60)], a 35 pack-year history of smoking, obstructive sleep apnea and hypercholesterolaemia and multifactorial anemia. The response to treatment was refractory.
Our patient has been one month with a positive result to the swab specimens by real-time reverse transcriptase-polymerase chain reaction. This is a Case Study with Long Term SARS-CoV-2. Our patient is significantly lethargic, confused, and disorientated, with seizure, altered mental state, headache, visual disturbances. Patient with SARS-CoV-2, severe pneumonia and cardiocirculatory arrest with pulseless electrical activity with CPR, Adrenaline and Revivan with achievement of restoration of spontaneous circulation (ROSC), endotracheal intubation; positive for Klebsiella oxytoca (2 consecutive samples): Trimethoprim sulfamethoxazole 400 mg/80 mg 3 vials x 4 daily. Eyes open with gaze to the right. Pain response in hyperextension and intra-rotation of upper limbs, stay in ‘decerebration’ attitude, vegetative state. Convulsive seizures being treated with Levetiracetam 1500 mg x 2 /day.
His blood examination displayed a total white cell count (WCC) of 11.5, a neutrophil count of 9.9 and a lymphocyte count of 0.6. His neutrophil-lymphocyte ratio (NLR) was 16.5 compared to expected value of <2 for his age group. C- reactive protein was also significantly elevated (132 mg/L compared to expected <5 mg/L) at admission. There was mild elevation in his creatinine levels from baseline. He was maintained on Moxonidine 200 mcg bid and his usual blood pressure medication, Prazosin 1 mg bid.
Computed tomography scan (CT scan) of his brain on admission showed bilateral hypo intensities around his posterior parietal-occipital regions. A subsequent cranial magnetic resonance taken on the same day revealed bilateral parieto-occipital hyperintensities compatible with Leukoencephalopathy given the recovering symptoms. There were diffuse petechial hemorrhages throughout the basal ganglia and deep white matter indicative of cerebral microbleeds. Multiple small foci of increased diffusion weighted imaging (DWI) signals with corresponding low apparent diffusion coefficient (ADC) signal were also noted in the deep white matter of the bilateral centrum semi oval and corona radiata (not shown as the changes are barely visible on the workstation console even). These could potentially be related to chronic hypertension, although possibilities involving acute COVID-19 related microangiopathy cannot be completely discounted. The electroencephalogram showed intermittent focal slow delta waves in posterior regions particularly in the left temporo-occipital region.
On October 10, 2020, our patient was computed tomography (CT) imaging of her chest a complete resolution of bilateral areas of altered density a ground glass after treatment. After six day our patient negative to Klebsiella and symptoms of Leukoencephalopathy (confused and disorientated, with seizure, altered mental state, headache, visual disturbances) are missing. Fortunately, On October 15, 2020, Nasopharyngeal swab specimens was negative and after the maintenance of intensive medical treatment in hospital computed tomography (CT) imaging of her chest a complete resolution (Figure 1).
Figure 1: Arterial Haemogasanalysis.
Note: Before Tocilizumab Therapy after therapy regression of pneumonia in patient with SARS-CoV-2 and Hypoxic Leukoencephalopathy.
Discussion
COVID-19 infection affected patients globally, with a high incidence in Europe and the Americas. The disease has affected patients of all age groups; however, heterogeneity in the outcome of COVID-19 infection associated with comorbidities, racial differences, and individual characteristics such as smoking has been observed [3]. Neurological manifestations associated with SARS-CoV-2 infection as well as its pathogenesis are insufficiently explained. Up to 36% of patients with COVID-19 have neurological manifestations, being more frequent in patients with greater clinical severity [4]. In critical patients with COVID-19, neurological symptoms usually involve several aetiological factors (such as sedation drugs, hypoxic and metabolic encephalopathy, encephalopathy induced by sepsis, and the potential neurological damage induced by the virus itself or by para-infectious mechanisms). Therefore, it is unlikely that a single isolated aetiological factor triggered the severe leukoencephalopathy of our patient.
Hypoxic Leukoencephalopathy is a clinicoradiological syndrome characterised by acute cerebral endotheliopathy with consecutive disruption of the blood-brain barrier and vasogenic oedema. Normal Clinical presentation of Leukoencephalopathy is based on at least one acute neurological symptom (seizure, altered mental state, headache, visual disturbances); one risk factor; brain imaging with bilateral vasogenic oedema, cytotoxic oedema or normal brain imaging; and no other alternative diagnosis. Fluctuations in blood pressure are a characteristic sign of Hypoxic Leukoencephalopathy both in SARS-CoV-2 patients and in other non-COVID associated diagnosis.
The link between Hypoxic Leukoencephalopathy and SARS-CoV-2 infection is not clear. Following SARS-CoV-2 infection, the expression and function of ACE 2 (ACE2) proteins are reduced, resulting in hypertension that may induce Encephalopathy [5]. Furthermore, vascular endothelial dysfunction has been recently proposed as complication of SARS-CoV-2 infection. SARS-CoV-2 infects the host using the ACE2 receptor, which is expressed in several organs and endothelial cells, resulting in diffuse endothelial inflammation. The SARS-CoV-2-infection-related endotheliitis could explain the systemic impaired microcirculatory function in different vascular beds. In the brain, this endotheliitis seems to induce increased permeability of the blood-brain barrier, leading to a brain oedema. Few cases of Leukoencephalopathy associated with COVID-19 have been published recently but are most often in severe forms requiring mechanical ventilation. Furthermore, there were other classical causes of PRES in these patients such as ARDS, tocilizumab, sepsis and acute kidney injury requiring haemodialysis, sever hypoxic pneumonia and treatment targeting SARS-CoV-2 that the authors could not determine the exact aetiology of Leukoencephalopathy [6, 7].
Our patient is characterised by the occurrence of Hypoxic Leukoencephalopathy, early in the course of the disease, in a non-severe form of SARS-CoV-2 infection with the need for respiratory assistance, cardiorespiratory arrest, cytokine storm syndrome, critical illness-related encephalopathy, multidrug regimens or metabolic disorders. That Encephalopathy could be considered as a complication of SARS-CoV-2 infection by means of this endotheliopathy. We need prospective studies of treatment options and additional patient characteristics to further understand the variables associated with COVID-19 associated death in patients with Hypoxic Leukoencephalopathy.
Conclusion
We can hope to provide adequate clinical care and urgently design interventional studies to prevent SARS-CoV-2 infection in the patient with Leukoencephalopathy because the mortality associated with this devastating pandemic is dramatically high. This is an exceptionally well-documented case of Hypoxic Leukoencephalopathy associated with a severe SARS-CoV-2 infection after cardiorespiratory arrest. Our observation adds further evidence that endotheliopathy related to SARS-CoV-2 infection may play a major role to cause Hypoxic Leukoencephalopathy. We advocate that timely Nuclear Magnetic Resonance is mandatory in suspected cases of Hypoxic Leukoencephalopathy especially in the context of confirmed SARS-CoV-2 infection to better characterise and manage this encephalopathy.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 10, May 2021Accepted: Sat 22, May 2021
Published: Mon 07, Jun 2021
Copyright
© 2023 Liliana Elena Weimer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.06.03
Author Info
Liliana Elena Weimer Cattari G Fanales Belasio E Binelli A Poddighe AF Sensi F
Corresponding Author
Liliana Elena WeimerCenter for Global Health, Istituto Superiore di Sanità, Rome, Italy
Figures & Tables
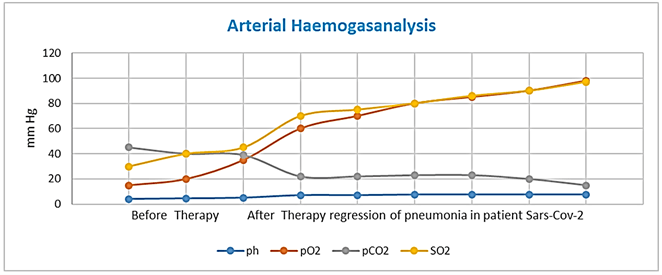
Note: Before Tocilizumab Therapy after therapy regression of pneumonia in patient with SARS-CoV-2 and Hypoxic Leukoencephalopathy.
References
1.
Zhou
P, Yang XL, Wang XG, Hu B, Zhang L et al. (2020) A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature 579: 270-273. [Crossref]
2. Li YC, Bai WZ, Hashikawa T (2020)
The
neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure
of COVID-19 patients. J Med Virol 92: 552-555. [Crossref]
3.
Martínez
López J, Mateos
MV, Encinas
C, Sureda A, Hernández Rivas JA et al. (2020) Multiple
myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic
factors of inpatient mortality. Blood Cancer J 10: 103. [Crossref]
4.
Zhou Z, Kang H, Li S, Zhao X (2020) Understanding the neurotropic characteristics
of SARS-CoV-2: from neurological manifestations of COVID-19 to potential
neurotropic mechanisms. J Neurol 267: 2179-2184. [Crossref]
5.
Varga Z, Flammer AJ,
Steiger P, Haberecker M, Andermatt R
et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417-1418. [Crossref]
6.
Kaya Y, Kara S,
Akinci C,
Kocaman AS (2020) Transient
cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: Case report. J Neurol Sci 413: 116858. [Crossref]
7.
Kishfy L, Casasola M,
Banankhah P,
Parvez A, Jan YJ et al. (2020) Posterior reversible encephalopathy
syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci 414: 116943. [Crossref]
