Journals
Long-term Efficacy and Safety of Donepezil Dose Escalation for Patients with Alzheimer?s Disease in a Clinical Setting
A B S T R A C T
Background: Donepezil is a routinely prescribed cognitive enhancer for patients with Alzheimer’s disease (AD), however the effectiveness and safety of long-term high doses remains largely unexplored.
Objective: We investigated the long-term efficacy and safety of Donepezil dose escalation in reducing global cognitive decline for patients with AD in a clinical setting.
Method: In a naturalistic, open-label, controlled study design, 71 mild to moderate AD patients from a tertiary clinic were prescribed Donepezil 5mg/day for 12 months (phase 1), while 9 AD patients received no treatment. Patients who showed limited benefits (N=30) with Donepezil 5mg/day were titrated up to 10mg/day for a subsequent 12 months (phase 2) and the remaining (N=41) patients continued on 5mg/day. The primary outcome was global cognition, indexed using the Mini-Mental State Examination (MMSE).
Results: Phase 1 trends confirmed Donepezil 5mg/day was better than no treatment at reducing cognitive decline (p = .09, f=.18). Phase 2 trends indicated that for patients who showed limited response to Donepezil 5mg/day, Donepezil 10mg/day was more effective in reducing slope of cognitive decline (p = 0.13, f= .42). Additionally, the patients that were titrated up to 10mg/day had comparable treatment benefits to those patients that remained on 5mg/day during phase 2 (p = .32, f =.12). Side effects in the 10mg/day group were not significantly different from the side effects in the 5mg group (t (67)=-1.27, p=.21).
Conclusion: Donepezil dose escalation in patients with AD is safe and may result in large noticeable effects on cognition, with effects comparable to patients who initially responded well to 5mg/day.
K E Y W O R D S
dementia, Alzheimer’s disease, treatment, efficacy, safety
I N T R O D U C T I O N
Alzheimer’s disease (AD) is the most common cause of dementia among the elderly. It is an irreversible, progressive neurodegenerative disorder, characterized by early indicators of cognitive decline, such as and memory deficits, that eventually progress to diminishing functional ability, behavioral disturbances and complete dependence on caregivers. Currently there is no cure for AD, however effective management strategies can delay the onset and progression of AD symptoms (ref). Such management has potential to reducing caregiver burden and enableling patients to maintain their independence and quality of life for longer.
One of the most successful management strategies to date has been altering the acetylcholine system in the brain. Degeneration of cholinergic neurons and reduced cholinergic neurotransmission has been proposed to underline symptoms of AD [1]. Donepezil is a cholinesterase inhibitor (ChEI) that has been proven to successfully increase acetylcholine levels and consequently cognitive functioning in patients with AD [2]. Subsequent dose escalation studies have shown that patients who experienced limited benefits from Donepezil 5mg/day appear to respond better when titrated up to 10mg/day [3]. What remains unclear is whether the patients who titrated up to 10mg/day experience the same strength of benefit on cognition as patients who initially responded will to 5mg/day.
We sought to identify the long-term effectiveness and safety of Donepezil dose escalation in reducing global cognitive decline in patients with AD compared to patients not receiving treatment and patients remaining on a lower dose of Donepezil. We hypothesized that AD patients who do not respond to 5mg/day and titrated up to 10mg/day may show comparable benefits to patients who initially responded to 5mg/day.
Methods
Study Design
This was a naturalistic, open-label, controlled study that retrospectively reviewed a clinical database from a tertiary neurology center, the National Neuroscience Institute (NNI) Singapore, between August 2008 and June 2016. The study was approved by the institutional Ethics Review Committee and informed consent was received from the patients themselves or their next of kin, prior to data collection.
Treatment schedule
The treatment group underwent two phases of treatment, each an average of 12 months in duration. During phase 1, all patients in the treatment group received a course of Donepezil 5mg/day, while the control group received no cognitive enhancer treatment. For phase 2, patients who were responding well to treatment continued on Donepezil 5mg/day (labeled here as the 5mg group), while patients who showed limited cognitive improvement during phase 1, as determined by neuropsychological tests and clinical judgment, were titrated up to Donepezil 10mg/day (labeled here as the 10mg group). Neuropsychological assessments were conducted at pre and post time points for phase 1 and phase 2.
Study Sample
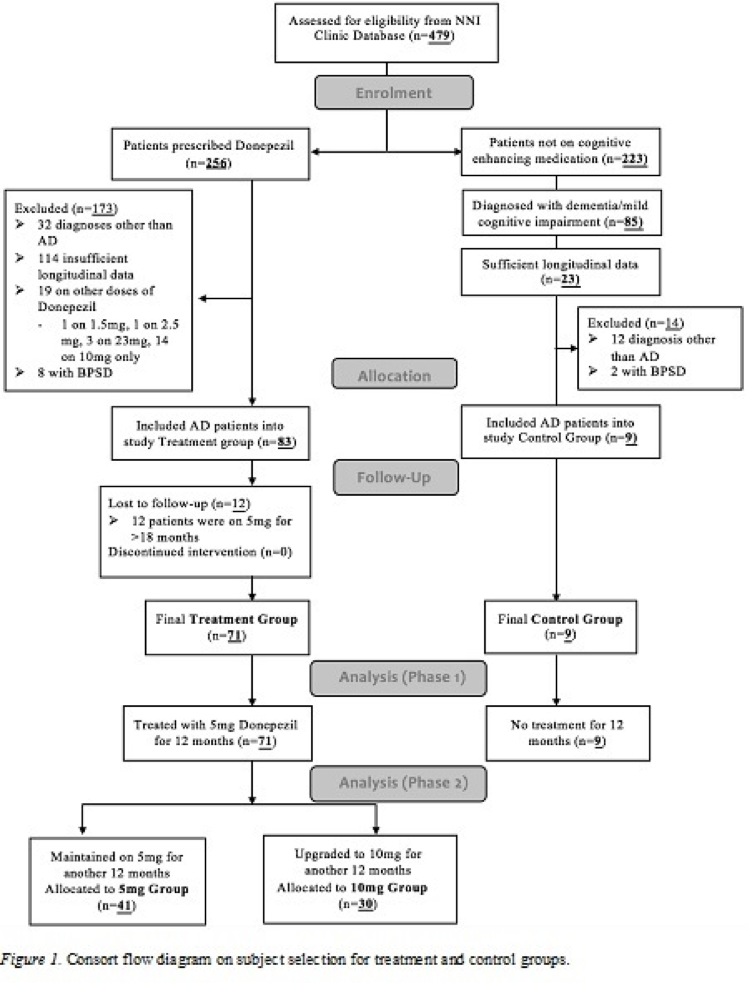
The clinical database included 479 patients with probable AD, diagnosed based on the National Institute of Ageing-Alzheimer’s Association Criteria (NIA-AA) Criteria [4]. Patients had a Clinical Dementia Rating Scale of 1- 3 [5]. Patients were allocated into the treatment group if they met the following criteria: 1) Prescribed Donepezil for at least 24 months, 2) availability of at least 3 neuropsychological assessments including baseline, 12 months and 24 months, and 3) no behavioral and psychological symptoms of dementia (BPSD) given that BPSD may be a potential confounder for cognitive functioning [6]. Patients were allocated into the control group if they met the following criteria: 1) not on any cognitive enhancer medication prior to or during the study period and 2) satisfied treatment group criteria 2 and 3. (Figure 1) illustrates the patient selection procedure for controls and treatment groups.
Measures
The primary outcome measure was change in global cognition, indexed using the Mini Mental State Examination (MMSE) [7]. The MMSE is a 30-point questionnaire that tests orientation, registration, attention and calculation, recall, and language. The MMSE is extensively used in dementia and drug research, providing a longitudinal “benchmark” of cognitive impairment [8].
A clinical interview was used to collect demographic information, including age, gender and years of education.
Statistical Methods
Statistical analyses were performed using SPSS version 20.0 [9].
I Group differences
Differences in baseline characteristics were assessed between controls and total treatment group, and between the 5mg group and 10mg group. A t-test was used for continuous variables and a chi test was used for categorical variables.
II Preliminary analysis
A preliminary analysis investigated whether a 12-month course of Donepezil treatment reduced the rate of decline on the MMSE compared to controls. Here, an ANOVA with repeated measures was used to measure change in MMSE scores, with time as the within-subjects factor and treatment type (Donepezil or control) as the between-subjects factor. A second preliminary analysis sought to confirm that patients in the treatment group who were titrated from 5mg/day to 10mg/day did indeed experience limited cognitive improvement with phase 1 5mg/day treatment. Here, an ANOVA with repeated measures was used to measure change in MMSE scores after 12 months of Donepezil 5mg/day, with time as the within-subjects factor and treatment group (5mg or 10mg group) as the between-subjects factor.
III Primary analysis
The primary analysis investigated whether increasing the Donepezil dose from 5mg/day to 10mg/day reduced the rate of decline on the MMSE. An ANOVA with repeated measures was used to evaluate change in MMSE scores, with dose and time as within-subject factors. Additionally, the rate of MMSE change for the 10mg group during phase 2 was compared to the rate of MMSE change for the 5mg group during phase 2. Here and ANOVA with repeated measures was used with phase 2 time as the within-factor and group (5mg group or 10mg group) as the between-subjects factor. All analyses controlled for covariates.
To determine the practical significance of our findings, we measured the effect size, which quantifies the degree to which the study results should be considered negligible or important regardless of the sample size and the original scales of the variables. Effect size indicates how important the effect is, while statistical significance indicates how strong the evidence is [11]. Effect size (Cohen’s f) was assessed using G*Power [12] and interpreted according to guidelines: f = 0.10 (small effect), f = 0.25 (moderate effect) and f = 0.40 (large effect) [13]. The statistical significance level for all analyses was set at p < .05, while p < .1 indicated a trend.
Figure 1:
Results
Study Sample
The study included 80 patients with mild to moderate AD, with 71 in the total treatment group and 9 in the control group. Figure 1 shows that during phase 1, all 71 patients in the total treatment group were allocated Donepezil 5mg/day, while during phase 2, 41 patients remained on 5mg/day and 30 patients titrated up to 10mg/day.
Group differences
Controls versus total treatment group: no differences were observed at baseline between controls and total treatment groups for age (t (78) = -.13, p >.05), years of education (t (77) = 1.02, p >.05) MMSE (t (78) = 1.35, p >.05), gender (x2 = (1,79) = .18, p >.05) or race (x2 = (2,79) = 4.59, p >.05).
5mg group versus 10mg Group: no differences were observed at baseline between the treatment group that stayed on 5mg/day and the group that escalated to 10mg/day during the second phase of treatment for age (t (69) = .13, p >.05), years of education (t (69) = -1.01, p >.05), MMSE (t (69) = -.48, p >.05), gender (x2 = (1,70) = .61, p >.05) or race (x2 = (2,70) = 4.37, p >.05).
Treatment group analysis
Preliminary analysis 1: Efficacy of Donepezil 5mg/day compared to controls
A repeated measures ANOVA with a Greenhouse-Geisser correction, controlling for covariates, indicated that the difference in MMSE score between the treatment group and control group was trending on significance (F (1,79) = 2.84, p = .09, f=.18). This indicates that Donepezil 5mg/day was trending on being significantly more effective at improving MMSE scores than no treatment, regardless of the effects of time. The change in MMSE scores between the treatment and control group was of a small to moderate effect size.
Preliminary analysis 2: Confirm whether patients titrated from Donepezil 5mg/day to 10mg/day during phase 2 was due to limited cognitive improvement at phase 1
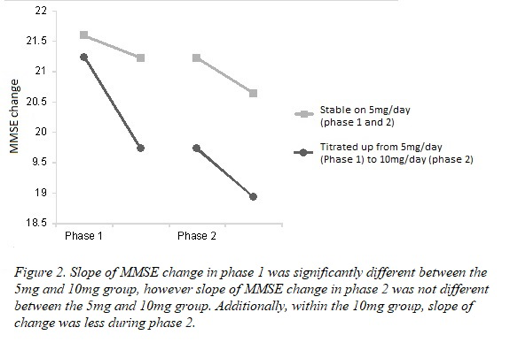
A repeated measures ANOVA with a Greenhouse-Geisser correction, controlling for covariates, indicated that there was a statistically significant interaction between time and treatment group on MMSE score (F (1,69) = 9.06, p = .00, f = .50). This indicates that despite all patients exhibiting similar MMSE scores at baseline, post phase 1 MMSE scores were significantly different between patients that remained on Donepezil 5mg/day for phase 2 and patients that were selected to titrate up to 10mg/day. Qualitative analysis indicated that the patients that remained on 5mg/day exhibited a limited decrease in MMSE scores post phase 1 (pre: 21.59 to post: 21.22), while patients that titrated up from 5mg/day to 10mg/day showed a large decline in MMSE score (pre: 21.23 to post: 19.73) post phase 1. The difference in post phase 1 scores between the two treatment groups was of a large effect size.
Primary analysis: Efficacy of Donepezil dose escalation
For patients in the 10mg treatment group, a repeated measures ANOVA with a Greenhouse-Geisser correction indicated that during phase 2 treatment, the change in MMSE scores was trending on being significantly smaller compared to the change in phase 1 (F (1, 29) = 5.10, p = 0.13, f= .42). This indicates that 10mg/day Donepezil was more effective at reducing cognitive decline than 5mg/day, as illustrated in Figure 2. This change in cognition resulting from dose escalation was of a large effect size.
When comparing change in MMSE scores between 5mg group and 10mg group during phase 2, a repeated measures ANOVA with a Greenhouse-Geisser correction, controlling for covariates, indicated that there was no significant interaction between time and dose on MMSE score, F (1,70) = .98, p = .32, f =.12. This indicates that change on the MMSE for those patients who remained on 5mg/day during phase 2 was not significantly different to the patients who titrated up to 10mg/day during phase 2, see Figure 2.
Figure 2:
Side Effects
A t-test indicated that when patients titrated up from 5mg/day to 10mg/day, the number of reported side effects increased (t (27) = -1.78, p =.09), an effect that was trending. Two patients in the 10mg/day group experienced side effects during phase 1 and continued to experience effects during phase 2 (one patient experienced occasional headaches while the other reported nausea and vomiting). An additional four patients in the 10mg/day group reported side effects during phase 2 (two experienced headaches, one reported trouble sleeping and one experienced weight-loss). In the 5mg group, two subjects report side-effects during phase 1 and 2, while four subjects reported side effects in phase 2. These side-effects were mild and included gastrointestinal side-effects including nausea and vomiting. Overall, the side effects in the 10mg group were not significantly different from the side effects in the 5mg group (t (67) =-1.27, p=.21).
Discussion
Main Findings
This study investigated the long-term efficacy and safety of Donepezil dose escalation in reducing global cognitive decline in patients with mild to moderate AD. A trend indicated that a 12-month course of Donepezil treatment (5mg/day) moderately reduced the rate of cognitive decline compared to no treatment, which supports previous findings on the benefit of Donepezil treatment in AD [14-16]. Despite this overall effect, some patients experienced limited cognitive gains on Donepezil 5mg/day, and their dose for phase 2 was increased to 10mg/day. This titration resulted in less cognitive decline compared to when they were receiving 5mg/day, an effect trending on significance. These therapeutic gains associated with a higher dose was consistent with previous studies [17, 3] and we extend these findings by demonstrating that when patients titrated up to 10mg/day, their response efficacy became equivalent with those patients who initially responded well to 5mg/day. We further demonstrated that long-term use and escalation from 5mg/day to 10mg/day was safe and well tolerated in patents with AD, with side-effects transient and manageable.
Donepezil 5mg/day had a moderate effect on cognitive decline compared to the no treatment group. This implies that noticeable changes in cognition may be observed after Donepezil treatment compared to those patients not taking treatment. Consistent with this finding, clinicians were correctly able to recognize treatment responders versus those who were not responding to 5mg/day and required a higher dose. For those patients who titrated up to a higher dose, the benefits of dose escalation were large, indicating that the change in treatment benefits may be easily observable. Previous findings have suggested that benefits of dose escalation may be marginal [2]. This difference may be associated with study design characteristics. For instance, Birks and colleagues reviewed 24 double-blind, randomized controlled trials in mild, moderate and severe Alzheimer's disease patients. As a result, their database may have had a larger portion of severe AD patients who were on the higher dose but did not respond well due to advanced progression of symptoms. Moreover, most trials were of 6 months or less in duration. Comparing these findings to the present study, we propose that the effects of Donepezil dose escalation may be larger in naturalistic observations with long-term Donepezil use in patients with mild to moderate AD, compared to RCTs with a short duration of treatment in patients with a wide range of symptom progression.
Clinical Implications
The practical relevance of these findings may guide clinicians to support early commencement and continuous treatment on a ChEI drug, as the stabilizing effects on cognitive decline remain effective after long term low and high dose Donepezil therapy. As the disease progresses, or in the absence of noticeable improvements from a low-dose of Donepezil, dose escalation would serve as a practical countermeasure for such problems in clinical practice. The present results may also ease potential concerns of continuous ChEI therapy desensitizing nicotinic acetylcholine receptors, leading to a decrease in the biological treatment response for AD symptoms [18].
Study strengths and limitations
Strengths of naturalistic studies include ecological and external validity, which confirm that the benefits of Donepezil treatment extend to the general population. As a result, our study compliments structured clinical trials and more pragmatic studies. Moreover, using a within subjects’ study design, we were able to evaluate the significance of each individual’s progress before and after dose escalation, as opposed to the majority of RCTs which focus on fixed treatment doses compared to placebo. A further strength included the long follow-up duration, which provided insight about tolerability and long-term effectiveness. We note several limitations with our naturalistic design, including lack of randomization, extraneous variables and observer bias. Furthermore, our data was gathered from a single center, which may limit the generalizability of our findings. We acknowledge that the preliminary finding of dose escalation efficacy was trending on significance; thus, our findings should be interpreted with caution and be corroborated with similar studies with a larger sample. However, we note that the effect sizes reported allow our findings to be comparable with other studies and our preliminary findings provide a valuable platform for future studies [19].
Conclusion
The present study demonstrated that dose escalation of Donepezil (5mg/day to 10mg/day) may result in large noticeable effects on cognition and may improve response efficacy to a rate that is equivalent to patients who initially respond well to 5mg/day. Donepezil dose escalation was well tolerated and thus remains to be an effective long-term pharmacological treatment for AD. Further studies are required with larger cohorts to confirm the effects of dose escalation.
Conflict of interest declaration
Associate Professor Nagaendran Kandiah has received honorarium and CME sponsorship from Lundbeck, Novartis, Pfizer, Schwabbe and Eisai. He has also received research funding from Singhealth Foundation, Media Development Authority of Singapore, Biomedical Research Council of Singapore and the National Medical Research Council of Singapore. CY, LT and DN have no conflicts of interest to declare.
Author Contributions
Dr Chathuri Yatawara, Laura Tan, and Associate Professor Nagaendran Kandiah were involved in study concept and design. Laura Tan and Debby Ng acquired the data. Dr Chathuri Yatawara conducted the analysis and interpretation of data. Dr Chathuri Yatawara and Laura Tan were involved in preparation of manuscript. Associate Professor Nagaendran Kandiah reviewed the manuscript for intellectual content.
Funding
This study was supported by the A*STAR Biomedical research council grant, Singapore, (13/1/96/19/687A).
Sponsor's Role: NA
Article Info
Article Type
Research ArticlePublication history
Received: Sat 28, Apr 2018Accepted: Wed 09, May 2018
Published: Fri 08, Jun 2018
Copyright
© 2023 Nagaendran Kandiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2018.10.004
Author Info
Laura Tan Chathuri J Yatawara Debby Ng Nagaendran Kandiah
Corresponding Author
Nagaendran KandiahDepartment of Neurology, National Neuroscience Institute, Singapore, Singapore
Figures & Tables
References
1. Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414. [Crossref]
2. Birks J, Harvey RJ (2006) Donepezil for dementia due to Alzheimer's disease. The Cochrane Library. [Crossref]
3. Yatabe Y, Hashimoto M, Kaneda K, Honda K, Ogawa Y, et al. (2013) Efficacy of increasing donepezil in mild to moderate Alzheimer's disease patients who show a diminished response to 5 mg donepezil: a preliminary study. Psychogeriatrics 13: 88-93. [Crossref]
4. Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8: 1-13. [Crossref]
5.Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43: 2412- 2414 [Crossref]
6. Fernández M, Gobartt AL, Balañá M (2010) Behavioural symptoms in patients with Alzheimer's disease and their association with cognitive impairment. BMC neurology 10: 87. [Crossref]
7. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198. [Crossref]
8. Salmon DP, Thal LJ, Butters N, Heindel WC (1990) Longitudinal evaluation of dementia of the Alzheimer type A comparison of 3 standardized mental status examinations. Neurology 40: 1225-1230. [Crossref]
9. SPSS I (2011) IBM SPSS statistics for Windows, version 20.0. New York: IBM Corp.
10. Wattmo C, Wallin AK, Londos E, Minthon L (2011) Predictors of long-term cognitive outcome in Alzheimer's disease. Alzheimers Res Ther 3: 23. [Crossref]
11. Sullivan GM, Feinn R (2012) Using effect size—or why the P value is not enough. J Grad Med Educ 4: 279-282. [Crossref]
12. Faul F, Erdfelder E, Lang AG, Buchner A (2007) G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191. [Crossref]
13. Ferguson CJ (2009) An effect size primer: A guide for clinicians and researchers. Professional Psychology: Res Practice 40: 532.
14. Burns A, Rossor M, Hecker J, Gauthier S, Petit H, et al. (1999) The Effects of Donepezil in Alzheimer’s Disease–Results from a Multinational Trial1. Dement Geriatr Cogn Disord 10: 237-244. [Crossref]
15. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology 50: 136-145. [Crossref]
16. Steele LS, Glazier RH (1999) Is donepezil effective for treating Alzheimer's disease? Can Fam Physician 45: 917-919. [Crossref]
17. Manabe Y, Ino T, Yamanaka K, Kosaka K (2016) Increased dosage of donepezil for the management of behavioural and psychological symptoms of dementia in dementia with Lewy bodies. Psychogeriatrics 16: 202-208. [Crossref]
18. Wang H, Sun X (2005) Desensitized nicotinic receptors in brain. Brain Res Brain Res Rev 48: 420-437. [Crossref]
19. Garamszegi LZ (2006) Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behavioral Ecology 17: 682-687 doi:10.1093/beheco/ark005.
