Long Term Outcome of Pulmonary Atresia with Major Aorto-Pulmonary Collaterals: Data from a Pooled Analysis
A B S T R A C T
Prognosis of Tetralogy of Fallot and Pulmonary atresia with MAPCAs is variable. We aimed to assess the outcome of patients who had undergone repair of TOF and PA with MAPCAs by different institutions and approaches, pooling together data on mortality and reinterventions. Abstracts were screened and full text papers were examined retrieving data on mortality, reinterventions and percentage of complete repair. Variables abstracted from the studies were pooled together and compared among different strategies. 28 papers out of 124 were selected, accounting for 1983 patients. 704 patients had undergone single stage unifocalization, 544 recruitment of PAs, 645 patients multistage and combined approaches. Median follow up was 49 months (36-70). 93 deaths occurred in the first month (5.4 %): 38 in group one (5.8%), 10 in group 2 (2.3%) and 45 in group 3 (8.6%), p<0.0001. At last follow up a total of 305 deaths occurred (16%) and did not differ among the three groups: 0.21 (0.15-0.25), 0.18 (0.07-0.26), 0.32 (0,13-0.38) deaths/100patients year, respectively p=0.43. 465 out of 969 patients, underwent at least one re-intervention (47.9%). Reintervention rate was similar among the three subsets and inversely correlated with the volume of institution (r= -0.45, p= 0.04). Although strategies of pulmonary atresia with MAPCAs repair are based on contrasting philosophies and extreme anatomic varibility, pooling together data from different studies, outcomes do not differ significantly and are characterized by a high rate of reinterventions. The latter appears to be significantly influenced by the volume of patients.
Keywords
Pulmonary atresia, MAPCAs, outcomes, management
Introduction
Pulmonary atresia and ventricular septal defect (PA-VSD) and tetralogy of Fallot (ToF) with major aortopulmonary collaterals (MAPCAs) account for the 25% of all PA [1]. This condition is characterized by hypoplastic or even absent central pulmonary arteries and pulmonary blood flow supplied to a variably extent by aortopulmonary vessels, probably derived from the splanchnic vascular plexus [2]. A great variability exists in terms of number of lung segments perfused by (MAPCAs), communication with native pulmonary circulation and development of intrapericardial pulmonary arteries. Initially, these patients were medically treated, thereafter, the anatomy of pulmonary tree was systematically studied by angiography and the concept of unifocalization of different sources of flow in a unique pathway has emerged [3]. The goal of the treatment is complete repair aiming at creating a single central PAs system and close the VSD with a low RV pressure [4].
Preliminary experiences of treatment by a staged inclusion of AP collaterals in a central system, were characterized by a significant mortality and high RV/LV pressure after VSD closure [5, 6]. Depending on center experience and anatomic variability, three main approaches have been devised: single stage unifocalization and complete repair, multistage focalization, and promotion of central arteries growth by either central shunt or right ventricle to pulmonary arteries connection. Unfocalization of all MPACAs and single stage repair has been demonstrated to be feasible and has emerged as first choice treatment based on the concept that the earlier AP collaterarals are unifocalized, the lower is the risk of segmental pulmonary vascular disease development [4]. However some concerns have been raised about the long term patency and development of reconstructed pulmonary arteries from collateral vessels [7].
Based on this background alternative approaches aiming at promoting native pulmonary arteries growth have been explored [8-20]. Published results of different strategies are contrasting in terms of mortality and reintervention rate, depending on the particular institution [7, 21-36]. One of the major limitation in the interpretation of long term results comparison is the overlap of surgical strategies in the treatment of a single patient, making it difficult to establish their relative contribution to the outcome [7]. Furthermore, most of the data about a specific approach come from a few high volume centers, raising concerns about their reproducibility. This study is a pooled analysis aiming at investigate the outcome of different strategies of ToF and PA with MAPCAs, repair. We particularly focused on total mortality, reintervention rate and growth of central pulmonary arteries for the three specific approaches.
Methods
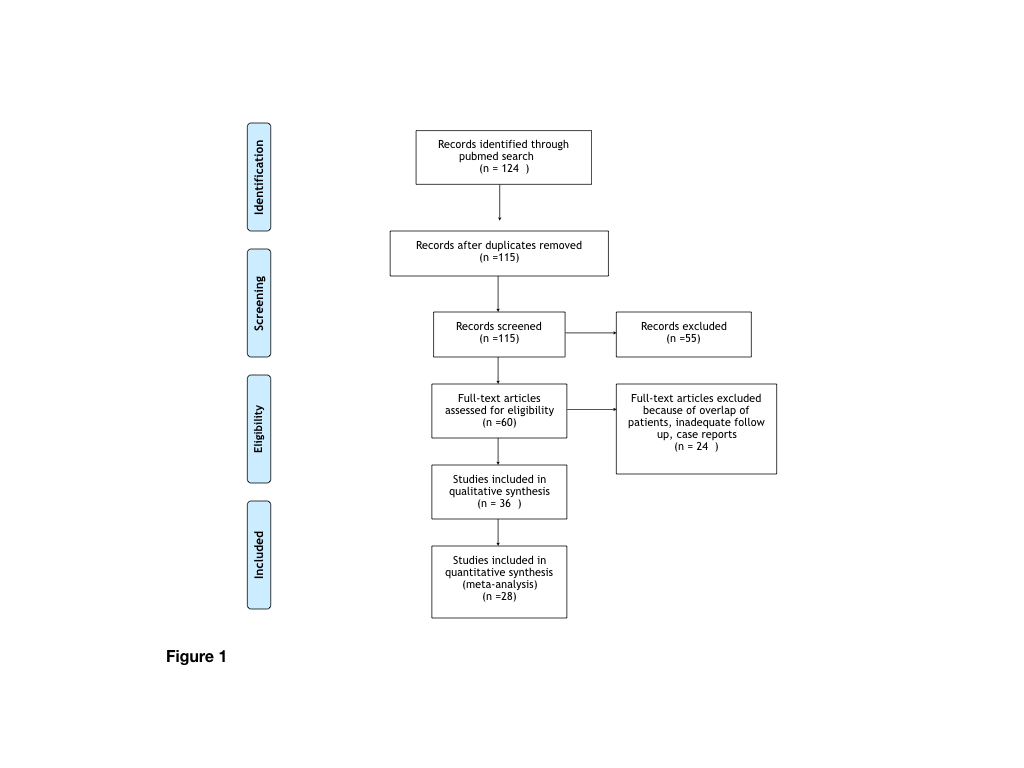
A systematic search of the literature on PubMed was performed using the following keywords: Pulmonary atresia, tetralogy of Fallot, MAPCAs. Paper screened were included according the following criteria: english language, clear information about mortality at follow up, publication after 2000. Case reports and case series not reporting long term follow up were also excluded. Sixty full text papers were examined and 28 of them were included in the present analysis. In the instance of papers from the same institution period of recruitment were checked in order to avoid duplication of data and the more recent dataset was considered (Figure 1). Surgical approaches were grouped into three categories: single stage unifocalization (Strategy 1), promotion of native pulmonary arteries growth by either central shunt or right ventricle to pulmonary arteries conduit (Strategy 2), and combined intervention including multistage focalization together with systemic-systemic-pulmonary shunts (Strategy 3).
Figure 1: Flowchart summarizing the selection of paper.
From each study the following data were recorded: type of strategy, early and late mortality, proportion of complete repair achievement, age and weight at complete repair, reintervention rate, right to left ventricular pressure and indexes of PAs growth. Reinterventions, including any procedure, either surgical or trans-catheter, performed after the initial intervention, not classified as postoperative complications or part of the staged algorithm.
Quantitative Analysis
Individual values of quantitative variables from each study were pooled together and summarized as median and interquartile range (25th-75th). In order to take into account different follow up lengths, mortality was expressed as number/100 patient month. Comparison of quantitative variables among the three strategies were performed by Kruskal Wallis test. Correlation between quantitative variables were explored by Sperman rank correlation test. P value of 0,05 was assumed as cut-off of significance.
Results
The search retrieved 124 items. Sixty full text papers were examined and 28 of them were included in the present analysis, accounting for a total of 1983 patients. Table 1 summarize demographic data of the pooled sample of patients. 704 patients had undergone single stage unifocalization and ventricular septal defect closure whenever possible, 544 patients had undergone recruitment of intrapericardial PAs by either central shunt or RV to PA conduit, 645 patients had undergone multistage unifocalization and combined approaches.
Table 1: Overall summary of the pooled studies.
|
Author |
Year |
Strategy |
N pts |
Early deaths |
Total deaths |
FU median (months) |
Complete repair, N (%) |
Age at repair median (days) |
Pts with at least one reintervention |
𝚫 Nakata |
Postrepair RV/LV |
|
Hofferberth et al. |
2018 |
1 |
41 |
2 |
6 |
58 |
22 (54) |
584 |
41 |
- |
0.65 |
|
|
|
3 |
43 |
4 |
9 |
68 |
15 (35) |
621 |
43 |
- |
0.82 |
|
Rabinowitz et al. |
2017 |
2 |
10 |
0 |
0 |
34 |
10 (100) |
239 |
10 |
130 |
<0.5 |
|
Trezzi et al. |
2017 |
1 |
95 |
8 |
21 |
102 |
64 (67) |
365 |
11 |
|
0.49 |
|
Lenoir et al. |
2017 |
2 |
109 |
3 |
10 |
60 |
84 (77) |
252 |
29 |
173 |
<0.5 |
|
Bauser-Heaton et al. |
2017 |
1 |
186 |
5 |
18 |
28 |
186 (100) |
162 |
- |
- |
0.35 |
|
|
|
2 |
46 |
2 |
4 |
37 |
35 (76) |
247 |
- |
- |
0.38 |
|
|
|
3 |
59 |
4 |
6 |
31 |
39 (66) |
367 |
- |
- |
0.39 |
|
Soquet et al. |
2016 |
1 |
4 |
0 |
0 |
55 |
4 (100) |
258 |
1 |
- |
- |
|
|
|
2 |
33 |
0 |
3 |
54 |
22 (67) |
617 |
13 |
- |
0.64 |
|
Ikeda et al. |
2015 |
3 |
13 |
1 |
2 |
13 |
9 (69) |
540 |
- |
- |
0.44 |
|
Kim et al. |
2015 |
2 |
15 |
0 |
2 |
70 |
13 (87) |
582 |
3 |
163.2 |
0.57 |
|
Watanabe et al. |
2014 |
1 |
27 |
0 |
2 |
48 |
27 (100) |
34 |
8 |
- |
0.35 |
|
Gerelli et al. |
2014 |
2 |
57 |
2 |
12 |
36 |
47 (82) |
10 |
26 |
121 |
- |
|
Fang et al. |
2014 |
2 |
103 |
0 |
0 |
11 |
66 (64) |
- |
- |
61 |
- |
|
Hibino et al. |
2013 |
2 |
23 |
0 |
3 |
44.7 |
20 (87) |
- |
17 |
225 |
|
|
Mainwaring et al. |
2012 |
2 |
35 |
0 |
29 |
49 |
18 (51) |
- |
35 |
- |
0.39 |
|
Liava et al. |
2012 |
2 |
20 |
0 |
0 |
39 |
12 (60) |
570 |
17 |
86.9 |
0.64 |
|
Dragulescu et al. |
2011 |
2 |
20 |
1 |
4 |
98.4 |
19 (95) |
744 |
19 |
182 |
0.58 |
|
Mei et al. |
2010 |
3 |
11 |
1 |
1 |
24 |
10 (91) |
- |
0 |
- |
- |
|
Murthy et al. |
2010 |
1 |
124 |
16 |
19 |
72 |
74 (60) |
1152 |
- |
- |
0.66 |
|
Honjo et al. |
2009 |
1 |
20 |
0 |
1 |
31 |
19 (95) |
234 |
10 |
- |
- |
|
Song et al. |
2009 |
3 |
40 |
2 |
7 |
54.5 |
17 (43) |
1080 |
4 |
115 |
0.57 |
|
Brizard et al. |
2009 |
2 |
15 |
0 |
0 |
- |
7 (47) |
600 |
- |
- |
0.59 |
|
Mumtaz et al. |
2008 |
2 |
40 |
2 |
4 |
68 |
25 (63) |
- |
13 |
149.3 |
- |
|
Ishibashi et al. |
2007 |
1 |
113 |
6 |
26 |
105.6 |
91 (81) |
243 |
- |
- |
0.7 |
|
Amark et al. |
2006 |
1 |
57 |
- |
26 |
45 |
57 (100) |
720 |
94 |
102 |
0.5 |
|
|
|
3 |
128 |
- |
28 |
45 |
82 (64) |
720 |
- |
74 |
- |
|
D’Udekem et al. |
2005 |
3 |
82 |
7 |
16 |
170.4 |
53 (65) |
1440 |
18 |
- |
0.62 |
|
Griselli et al. |
2004 |
1 |
37 |
1 |
4 |
144 |
37 (100) |
- |
41 |
- |
0.6 |
|
|
|
3 |
119 |
14 |
22 |
- |
89 (75) |
- |
- |
- |
- |
|
Duncan et al. |
2003 |
3 |
46 |
0 |
1 |
44 |
28 (61) |
1050 |
14 |
- |
0.45 |
|
Gupta et al. |
2003 |
3 |
104 |
12 |
17 |
122 |
58 (56) |
1872 |
19 |
- |
0.5 |
|
Rodefeld et al. |
2002 |
2 |
18 |
0 |
2 |
- |
11 (61) |
138 |
- |
- |
- |
|
Pooled |
|
|
1893 |
93 |
305 |
48 |
1370 (72) |
570 |
475 |
121 |
0.54 |
The median number of patients scheduled for the three different procedure was not significantly different: 57 (27-113); 28 (1-46); 52 (40-104) respectively, p=0.14 (Table 2). The choice among the three strategies tended to aggregate in each institution. Early and late death rate were missing in two and four studies, respectively. Median follow up was not stated in 3 studies. Age at repair, total reintervention rate and RV/LV ratio was not available in 7, 11 and 10 studies, respectively (Table 3).
Table 2: Comparison among strategies.
|
|
Overall |
Single stage unifocalization |
Pulmonary arteries recruitment |
Multi-stage unifocalization/ mixed |
P value |
|
N, median ( 25th-75th) |
40.5 (20-95) |
57 (27-113) |
28 (18-46) |
52 (40-104) |
0.14 |
|
Follow up (months), median ( 25th-75th ) |
49 (36-70) |
56.5 (45-102) |
46.8 (36-64) |
45 (31-68) |
0.5 |
|
Age at repair (days), median ( 25th-75th ) |
570 (243-720) |
258 (234-584) |
411 (239-600) |
885 (580-1260) |
0.02 |
|
Complete repair (%), median ( 25th-75th ) |
68 (61-91) |
97 (67-100) |
71 (61-87) |
64 (56-69) |
0.04 |
|
Death/ 100 pts/ months, median ( 25th-75th ) |
0.21 (0.13-0.32) |
0.21 (0.15-0.25) |
0.18 (0.07-0.26) |
0.32 (0.13-0.38) |
0.43 |
|
Reintervention/ 100 pts/ months, median ( 25th-75th ) |
0.73 (0.44-1.61) |
0.69 (0.45-1.61) |
0.87 (0.48-2) |
0.18 (0.15-0.69) |
0.1 |
|
RV/ LV pressure, median ( 25th-75th ) |
0.53 (0.44-0.63) |
0.5 (0.41-0.63) |
0.57 (0.5-0.6) |
0.5 (0.44-0.62) |
0.91 |
Table 3: Distribution of missing data among different strategies.
|
Author |
Year |
N patients |
Type of procedure |
|
Hofferberth et al. |
2018 |
43 |
Multistage unifocalization |
|
Bauser-Heaton et al. |
2017 |
59 |
Unifocalization to shunt |
|
Ikeda et al. |
2015 |
13 |
Unifocalization and staged VSD closure |
|
Mei et al. |
2010 |
11 |
Unifocalization to shunt |
|
Song et al. |
2009 |
40 |
MBT shunt / unifocalization to shunt |
|
Amark et al. |
2006 |
105 |
MBT shunt |
|
|
|
23 |
RVOT recostruction |
|
D’Udekem et al. |
2005 |
82 |
Central shunt + unifocalization |
|
Griselli et al. |
2004 |
119 |
Multistage unifocalization |
|
Duncan et al. |
2003 |
46 |
Central shunt + unifocalization |
|
Gupta et al. |
2003 |
104 |
Multistage unifocalization |
Operative Data
Patients included in group 1 underwent single stage unifocalization of all suitable MAPCAs and VSD closure whenever possible. Decision to close the VSD relied on the following criteria: intraoperative flow study and RV/LV pressure ratio measurement and surgeon judgement of the completeness of pulmonary vascular tree. Patients within group 2 underwent primarily right ventricular outflow reconstruction or central shunt in order to promote native PAs growth. Within this subset a significant growth of central pulmonary arteries was observed with Nakata index median increase of 121 mm2/m2 (74-182).
Under strategy 3, are grouped patients who underwent multistaged unifocalization (389 patients), central shunt and unifocalization (128 patients), MBT shunt and RVOT reconstruction ( 128 patients) (Table 4). In the whole population 68% (61-91) of patients underwent complete repair. Patients scheduled for multistage/combined strategy underwent complete repair at a significant older age as compared with strategy 1 and 2, 885 (580-1260) days versus 258 (234-584) and 411 (239-600), respectively, p=0.02 (Figure 2B). In group one 97% (67-100) had the VSD closed during the period of study, vs 71% (61-87), for strategy 2 and 64%(56-69), for strategy 3, p=0,04 (Figure 2A). Percentage of successful repair inversely correlated with age at repair: r= -0.56, p=0.02. (Figure 2D). Median right/left ventricle pressure ratio after VSD closure was 0.53(0.44-0.63) and did not differed among strategies: 0.5 (0.41-0.63), 0.57 (0.5-0.6) and 0.5 (0.44-0.62), respectively, p=0.9 (Table 2).
Table 4: Detailed procedures performed in strategy n.3.
|
Missing variables: N (%) |
Strategy n.1 |
Strategy n.2 |
Strategy n.3 |
|
Early deaths |
1 (10%) |
0 |
1 (10%) |
|
Late deaths |
2 (20%) |
0 |
2 (20%) |
|
Follow-up |
0 |
2 (14%) |
1 (10%) |
|
VSD closure % |
0 |
0 |
0 |
|
Age at repair |
1 (10%) |
4 (29%) |
2 (20%) |
|
Total reintervention rate |
3 (30%) |
4 (29%) |
4 (40%) |
|
RV / LV ratio |
2 (20%) |
5 (36%) |
3 (30%) |
Figure 2: A) Box-plot illustrating differences of pooled percentage of complete repair among the three different strategies, B) Box-plot illustrating pooled median age at repair among the three different strategies, C) Scatter-plot showing correlation between mortality, reintervention rate and patient’s sample size, D) Scatter-plot showing correlation between percentage of repair and median age at repair.
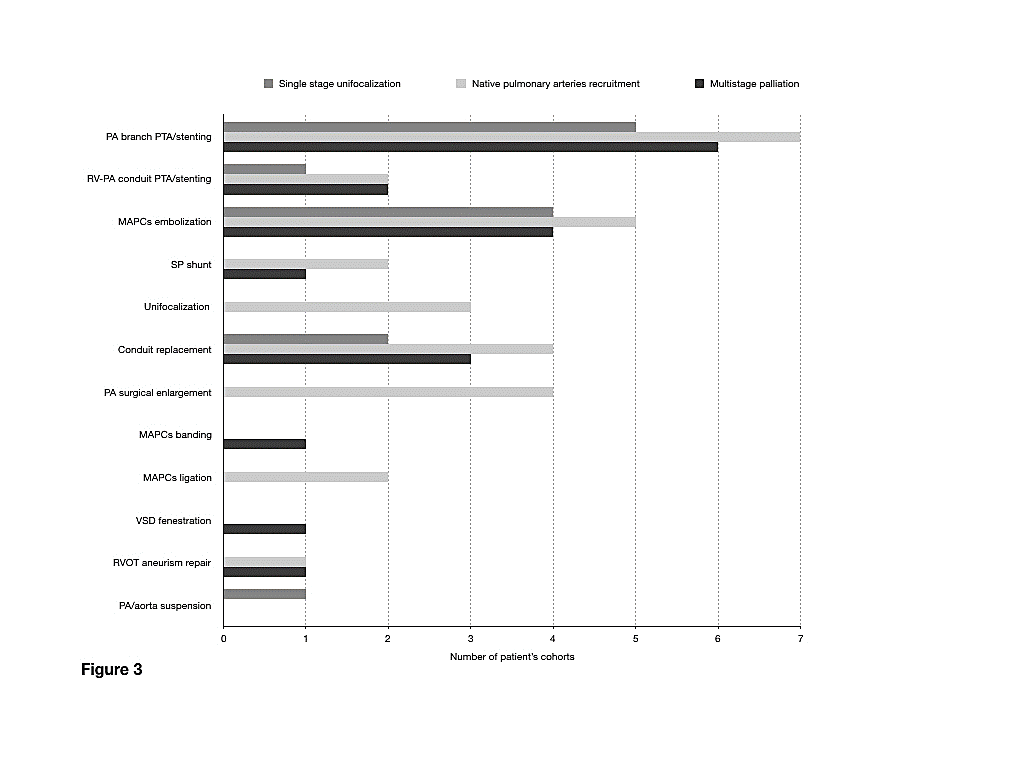
Figure 3: Diagram depicting distribution of reintervention type among the three different strategies.
Outcomes
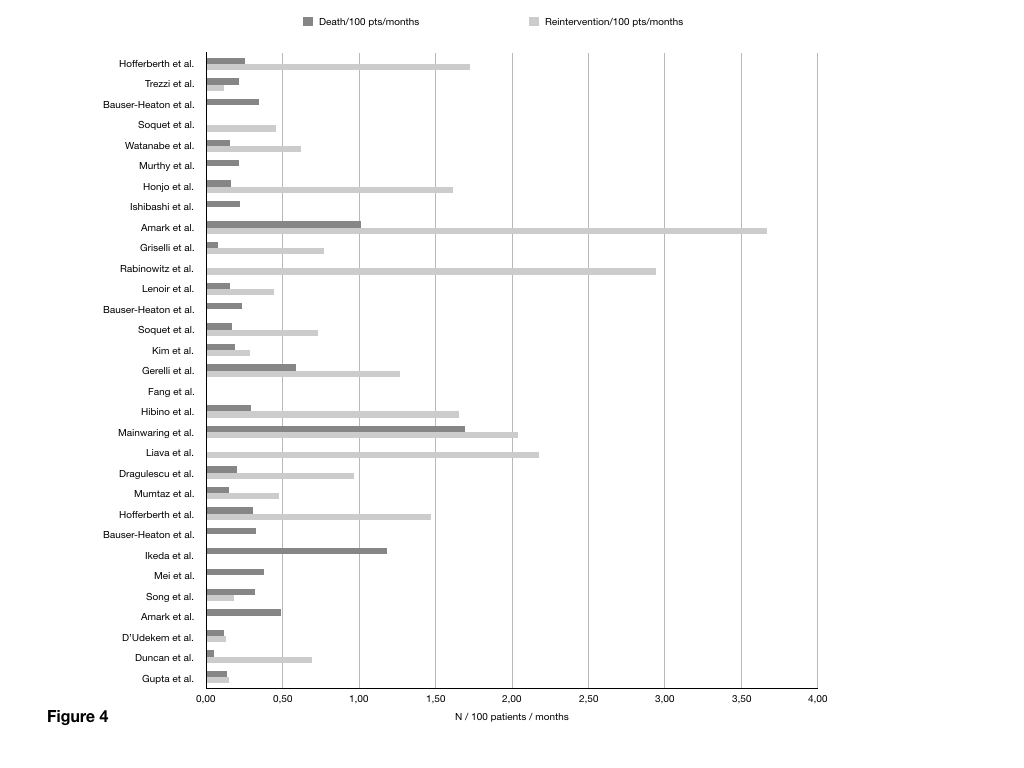
Median follow up for the whole pooled population was 49 months (36-70) and it was similar among the three strategies: 56.5 (45-102), 46.8 (36-64), 45 (31-68) months, respectively, p=0.5. Overall 93 deaths occurred in the first month ( 5.4 %): 38 in group one (5.8%), 10 in group 2 (2.3%) and 45 in group 3 (8.6%), respectively p<0.0001. Figure 3 summarizes mortality and reintervention rate distribution for each study. Overall, at last follow up a total of 305 deaths occurred, accounting for a total mortality rate of 0.21 (0.13-0.32) death/100 patients year and did not differ among the three groups: 0.21 (0.15-0.25), 0.18 (0.07-0.26), 0.32 (0,13-0.38) deaths/100patients year (p=0.43).
At last follow up a total of 465 out of 969 patients, underwent at least one intervention (47.9%), giving a total reintervention rate of 0.73(0.44-1.61) /100 patients year. Catheter based interventions addressed pulmonary arteries ( n=18 studies), RV to PAs conduit (n=5 studies), and MAPCAs (n=13 studies). Surgical reinvention addressed the conduit and pulmonary arteries in 9 and 4 studies, respectively and MAPCAs in three cases. VSD fenestration, RVOT aneurism repair and pulmonary artery or aorta resuspension were reported in one, two and one study, respectively (Figure 4).
Figure 4: Bar-chart illustrating mortality and reintervention rate for each individual study.
No significant differences in reintervention rate were found: 0.69 (0,45-1.61) /100 pts months for strategy 1, 0.87(0.48-2) for strategy 2 and 0.18 (0.15-0.69) for strategy 3 (Table 2). Reintervention rate was inversely correlated with the number of patients enrolled, r= -0.45 (p=0.04), while no association was observed between overall mortality and sample size (Figure 2C). Data on development of pulmonary vascular disease were reported in nine papers providing a median percentage of 10% (1-12).
Discussion
Repair of Tetralogy of Fallot and pulmonary atresia with major aortopulmonary collaterals present many decisional and surgical challenges as anatomy is usually characterized by severely or even absent central PAs and pulmonary blood supply is granted by systemic collaterals. Some groups have made attempts to provide a decisional algorithm in order to standardize the treatment according to the type of anatomy (Figure 5) [24]. A mainstay of the surgical treatment is recruitment of as many collaterals as possible to create a low resistance pulmonary pathway, based on the concept that collateral vessels may grow like native PAs. This notion has been challenged by d’Udekem et al. who reported the lack of proportional increase of the cross sectional area of MPCAs derived vessels [7]. Based on this concept, an opposite approach aiming at promoting native PAs development has been devised. According to this approach, MAPCAs are not utilized unless they represent the unique pulmonary supply of a lung segment, relying on the genetic commitment of PAs to grow under the proper stimulus of flow [8-20].
This philosophy is supported by the observation of a significant increase in Nakata index that was confirmed in our analysis. However, within group two, no correlation between magnitude of PAs growth and mortality, reintervention rate and percentage of complete repair was observed. Patients candidate for this approach had a lower in hospital mortality, likely explained by a more favorable anatomy due to the presence of intrapericardial confluent PAs. Despite the plausibility of the pathophysiologic advantage of promoting native PAs growth over using collateral vessels, gathering together all studies, we did not observe any difference in terms of total long term mortality reintervention rate and percentage of repair among the three strategies. This finding might be explained by a possible selection bias, as the choice among the different strategies is mostly institution dependent, thus patients considered for one of the three approach are those most suitable in term of anatomy.
Interestingly, age at repair inversely correlated with percentage of repair, meaning that the more time elapses from initial palliation the lower is the likelihood of successful complete repair. The lack of significant differences in the long term outcome among different strategies suggests that many factors, beside creation of an adequate central pulmonary pathway, play a role in the final vascular run-off. One of the main cofactors affecting the long term prognosis is the development of pulmonary vascular disease whose estimated median incidence in our pooled cohort was particularly high. Unfortunately due to the high percentage of missing data we were not able to explore any differences among groups.
Despite a low mortality rate, all three strategies are burdened by a significant rate of reinterventions during follow up, addressing both the RV-PA conduits the PAs and MAPCAs. These were equally distributed among the three groups, suggesting that branch pulmonary arteries morbidity is significant, irrespective of type of initial surgery. We might speculate that despite adequate central PAs growth, the presence of competitive flow from MAPCAs may cause underdevelopment and stenosis in the distal segments, which in turn impact on the final outcome. On the other hand, this finding confirm the concept that MAPCAs are prone to develop narrowing during follow up. The reintervention rate inversely correlates with the center volume, that appears as only determinant of the long term reintervention rate in the whole population.
Limitations
The main limitation of this systematic review is the overlap among group of patients scheduled for different strategies, limiting the possibility to allocate properly the outcomes. As variable values that could not be univocally associated with a specific strategy were excluded, the number of missing data was not negligible. Furthermore almost all studies included in this analysis are retrospective. Finally most of data come from few high volume center, introducing a possible bias in the comparison of outcomes.
Figure 5: Diagram depicting different kind of palliation strategy.
Conclusion
Despite different approaches, based on opposite philosophies, have been developed to manage ToF/PA and MAPCAs, we did not find any significant difference in term of outcome. Based on available data of the literature no general algorithm of treatment can be recommended according to a clear superiority of a treatment philosophy. The only variable associated with the outcome was the volume of the center. The choice of the best strategy should be tailored on individual anatomy, physiology and expertise of the center.
Conflicts of Interest
None.
Acknowledgement
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 06, Apr 2020Accepted: Mon 20, Apr 2020
Published: Fri 24, Apr 2020
Copyright
© 2023 Paolo Ferrero. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.02.08
Author Info
Isabelle Piazza Matteo Ciuffreda Paolo Ferrero Youcef Sadou
Corresponding Author
Paolo FerreroPediatric Cardiology Division, Cardiovascular Department, ASST Papa Giovanni XXIII, Bergamo, Italy
Figures & Tables
Table 1: Overall summary of the pooled studies.
|
Author |
Year |
Strategy |
N pts |
Early deaths |
Total deaths |
FU median (months) |
Complete repair, N (%) |
Age at repair median (days) |
Pts with at least one reintervention |
𝚫 Nakata |
Postrepair RV/LV |
|
Hofferberth et al. |
2018 |
1 |
41 |
2 |
6 |
58 |
22 (54) |
584 |
41 |
- |
0.65 |
|
|
|
3 |
43 |
4 |
9 |
68 |
15 (35) |
621 |
43 |
- |
0.82 |
|
Rabinowitz et al. |
2017 |
2 |
10 |
0 |
0 |
34 |
10 (100) |
239 |
10 |
130 |
<0.5 |
|
Trezzi et al. |
2017 |
1 |
95 |
8 |
21 |
102 |
64 (67) |
365 |
11 |
|
0.49 |
|
Lenoir et al. |
2017 |
2 |
109 |
3 |
10 |
60 |
84 (77) |
252 |
29 |
173 |
<0.5 |
|
Bauser-Heaton et al. |
2017 |
1 |
186 |
5 |
18 |
28 |
186 (100) |
162 |
- |
- |
0.35 |
|
|
|
2 |
46 |
2 |
4 |
37 |
35 (76) |
247 |
- |
- |
0.38 |
|
|
|
3 |
59 |
4 |
6 |
31 |
39 (66) |
367 |
- |
- |
0.39 |
|
Soquet et al. |
2016 |
1 |
4 |
0 |
0 |
55 |
4 (100) |
258 |
1 |
- |
- |
|
|
|
2 |
33 |
0 |
3 |
54 |
22 (67) |
617 |
13 |
- |
0.64 |
|
Ikeda et al. |
2015 |
3 |
13 |
1 |
2 |
13 |
9 (69) |
540 |
- |
- |
0.44 |
|
Kim et al. |
2015 |
2 |
15 |
0 |
2 |
70 |
13 (87) |
582 |
3 |
163.2 |
0.57 |
|
Watanabe et al. |
2014 |
1 |
27 |
0 |
2 |
48 |
27 (100) |
34 |
8 |
- |
0.35 |
|
Gerelli et al. |
2014 |
2 |
57 |
2 |
12 |
36 |
47 (82) |
10 |
26 |
121 |
- |
|
Fang et al. |
2014 |
2 |
103 |
0 |
0 |
11 |
66 (64) |
- |
- |
61 |
- |
|
Hibino et al. |
2013 |
2 |
23 |
0 |
3 |
44.7 |
20 (87) |
- |
17 |
225 |
|
|
Mainwaring et al. |
2012 |
2 |
35 |
0 |
29 |
49 |
18 (51) |
- |
35 |
- |
0.39 |
|
Liava et al. |
2012 |
2 |
20 |
0 |
0 |
39 |
12 (60) |
570 |
17 |
86.9 |
0.64 |
|
Dragulescu et al. |
2011 |
2 |
20 |
1 |
4 |
98.4 |
19 (95) |
744 |
19 |
182 |
0.58 |
|
Mei et al. |
2010 |
3 |
11 |
1 |
1 |
24 |
10 (91) |
- |
0 |
- |
- |
|
Murthy et al. |
2010 |
1 |
124 |
16 |
19 |
72 |
74 (60) |
1152 |
- |
- |
0.66 |
|
Honjo et al. |
2009 |
1 |
20 |
0 |
1 |
31 |
19 (95) |
234 |
10 |
- |
- |
|
Song et al. |
2009 |
3 |
40 |
2 |
7 |
54.5 |
17 (43) |
1080 |
4 |
115 |
0.57 |
|
Brizard et al. |
2009 |
2 |
15 |
0 |
0 |
- |
7 (47) |
600 |
- |
- |
0.59 |
|
Mumtaz et al. |
2008 |
2 |
40 |
2 |
4 |
68 |
25 (63) |
- |
13 |
149.3 |
- |
|
Ishibashi et al. |
2007 |
1 |
113 |
6 |
26 |
105.6 |
91 (81) |
243 |
- |
- |
0.7 |
|
Amark et al. |
2006 |
1 |
57 |
- |
26 |
45 |
57 (100) |
720 |
94 |
102 |
0.5 |
|
|
|
3 |
128 |
- |
28 |
45 |
82 (64) |
720 |
- |
74 |
- |
|
D’Udekem et al. |
2005 |
3 |
82 |
7 |
16 |
170.4 |
53 (65) |
1440 |
18 |
- |
0.62 |
|
Griselli et al. |
2004 |
1 |
37 |
1 |
4 |
144 |
37 (100) |
- |
41 |
- |
0.6 |
|
|
|
3 |
119 |
14 |
22 |
- |
89 (75) |
- |
- |
- |
- |
|
Duncan et al. |
2003 |
3 |
46 |
0 |
1 |
44 |
28 (61) |
1050 |
14 |
- |
0.45 |
|
Gupta et al. |
2003 |
3 |
104 |
12 |
17 |
122 |
58 (56) |
1872 |
19 |
- |
0.5 |
|
Rodefeld et al. |
2002 |
2 |
18 |
0 |
2 |
- |
11 (61) |
138 |
- |
- |
- |
|
Pooled |
|
|
1893 |
93 |
305 |
48 |
1370 (72) |
570 |
475 |
121 |
0.54 |
Table 2: Comparison among strategies.
|
|
Overall |
Single stage unifocalization |
Pulmonary arteries recruitment |
Multi-stage unifocalization/ mixed |
P value |
|
N, median ( 25th-75th) |
40.5 (20-95) |
57 (27-113) |
28 (18-46) |
52 (40-104) |
0.14 |
|
Follow up (months), median ( 25th-75th ) |
49 (36-70) |
56.5 (45-102) |
46.8 (36-64) |
45 (31-68) |
0.5 |
|
Age at repair (days), median ( 25th-75th ) |
570 (243-720) |
258 (234-584) |
411 (239-600) |
885 (580-1260) |
0.02 |
|
Complete repair (%), median ( 25th-75th ) |
68 (61-91) |
97 (67-100) |
71 (61-87) |
64 (56-69) |
0.04 |
|
Death/ 100 pts/ months, median ( 25th-75th ) |
0.21 (0.13-0.32) |
0.21 (0.15-0.25) |
0.18 (0.07-0.26) |
0.32 (0.13-0.38) |
0.43 |
|
Reintervention/ 100 pts/ months, median ( 25th-75th ) |
0.73 (0.44-1.61) |
0.69 (0.45-1.61) |
0.87 (0.48-2) |
0.18 (0.15-0.69) |
0.1 |
|
RV/ LV pressure, median ( 25th-75th ) |
0.53 (0.44-0.63) |
0.5 (0.41-0.63) |
0.57 (0.5-0.6) |
0.5 (0.44-0.62) |
0.91 |
Table 3: Distribution of missing data among different strategies.
|
Author |
Year |
N patients |
Type of procedure |
|
Hofferberth et al. |
2018 |
43 |
Multistage unifocalization |
|
Bauser-Heaton et al. |
2017 |
59 |
Unifocalization to shunt |
|
Ikeda et al. |
2015 |
13 |
Unifocalization and staged VSD closure |
|
Mei et al. |
2010 |
11 |
Unifocalization to shunt |
|
Song et al. |
2009 |
40 |
MBT shunt / unifocalization to shunt |
|
Amark et al. |
2006 |
105 |
MBT shunt |
|
|
|
23 |
RVOT recostruction |
|
D’Udekem et al. |
2005 |
82 |
Central shunt + unifocalization |
|
Griselli et al. |
2004 |
119 |
Multistage unifocalization |
|
Duncan et al. |
2003 |
46 |
Central shunt + unifocalization |
|
Gupta et al. |
2003 |
104 |
Multistage unifocalization |
Table 4: Detailed procedures performed in strategy n.3.
|
Missing variables: N (%) |
Strategy n.1 |
Strategy n.2 |
Strategy n.3 |
|
Early deaths |
1 (10%) |
0 |
1 (10%) |
|
Late deaths |
2 (20%) |
0 |
2 (20%) |
|
Follow-up |
0 |
2 (14%) |
1 (10%) |
|
VSD closure % |
0 |
0 |
0 |
|
Age at repair |
1 (10%) |
4 (29%) |
2 (20%) |
|
Total reintervention rate |
3 (30%) |
4 (29%) |
4 (40%) |
|
RV / LV ratio |
2 (20%) |
5 (36%) |
3 (30%) |


References
- Kirklin JW, Barratt Boyes BG (1993) Tetralogy of Fallot with pulmonary atresia in Kirklin JW, Barratt Boyes BG (eds). Cardiac surgery 2nd ed. New York: Churchill Livingstone 1993: 942-973.
- DeRuiter MC, Gittenberger de Groot AC, Poelmann RE, VanIperen L, Mentink MM (1993) Development of the pharyngeal arch system related to the pulmonary and bronchial vessels in the avian embryo: With a concept on systemic-pulmonary collateral artery formation. Circulation 87: 1306-1319. [Crossref]
- Haworth SG, Macartney FJ (1980) Growth and development of pulmonary circulation in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Br Heart J 44: 14-24. [Crossref]
- Reddy VM, Liddicoat JR, Hanley FL (1995) Midline one-stage complete unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 109: 832-844. [Crossref]
- Puga FJ, Leoni FE, Julsrud PR, Mair DD (1989) Complete repair of pulmonary atresia, ventricular septal defect, and severe peripheral arborization abnormalities of the central pulmonary arteries. Experience with preliminary unifocalization procedures in 38 patients. J Thorac Cardiovasc Surg 98: 1018-1029. [Crossref]
- Iyer KS, Mee RB (1991) Staged repair of pulmonary atresia with ventricular septal defect and major systemic to pulmonary artery collaterals. Ann Thorac Surg 51: 65-72. [Crossref]
- d'Udekem Y, Alphonso N, Nørgaard MA, Cochrane AD, Grigg LE et al. (2005) Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries: unifocalization brings no long-term benefits. J Thorac Cardiovasc Surg 130: 1496-1502. [Crossref]
- Rabinowitz EJ, Epstein S, Kohn N, Meyer DB (2017) Promoting Pulmonary Arterial Growth via Right Ventricle-to-Pulmonary Artery Connection in Children With Pulmonary Atresia, Ventricular Septal Defect, and Hypoplastic Pulmonary Arteries. World J Pediatr Congenit Heart Surg 8: 564-569. [Crossref]
- Lenoir M, Pontailler M, Gaudin R, Gerelli S, Tamisier D et al. (2017) Outcomes of palliative right ventricle to pulmonary artery connection for pulmonary atresia with ventricular septal defect. Eur J Cardiothorac Surg 52: 590-598. [Crossref]
- Gerelli S, van Steenberghe M, Murtuza B, Bojan M, Harding ED et al. (2014) Neonatal right ventricle to pulmonary connection as a palliative procedure for pulmonary atresia with ventricular septal defect or severe tetralogy of Fallot. Eur J Cardiothorac Surg 45: 278-288. [Crossref]
- Murthy K, Reddy KP, Nagarajan R, Goutami V, Cherian K (2010) Management of ventricular septal defect with pulmonary atresia and major aorto pulmonary collateral arteries: Challenges and controversies. Ann Pediatr Cardiol 3: 127-135. [Crossref]
- Brizard CP, Liava'a M, d'Udekem Y (2009) Pulmonary atresia, VSD and Mapcas: repair without unifocalization. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2009: 139-144. [Crossref]
- Rodefeld MD, Reddy VM, Thompson LD, Suleman S, Moore PC et al. (2002) Surgical creation of aortopulmonary window in selected patients with pulmonary atresia with poorly developed aortopulmonary collaterals and hypoplastic pulmonary arteries. J Thorac Cardiovasc Surg 123: 1147-1154. [Crossref]
- Fang M, Wang H, Jin Y, Wang Z, Wang Z et al. (2014) Development of pulmonary arteries after a central end-to-side shunt in patients with pulmonary atresia, ventricular septal defect, and diminutive pulmonary arteries. J Thorac Cardiovasc Surg 62: 211-215. [Crossref]
- Liava'a M, Brizard CP, Konstantinov IE, Robertson T, Cheung MM et al. (2012) Pulmonary atresia, ventricular septal defect, and major aortopulmonary collaterals: neonatal pulmonary artery rehabilitation without unifocalization. Ann Thorac Surg 93: 185-191. [Crossref]
- Dragulescu A, Kammache I, Fouilloux V, Amedro P, Métras D et al. (2011) Long-term results of pulmonary artery rehabilitation in patients with pulmonary atresia, ventricular septal defect, pulmonary artery hypoplasia, and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 142: 1374-1380. [Crossref]
- Mainwaring RD, Reddy VM, Perry SB, Peng L, Hanley FL (2012) Late outcomes in patients undergoing aortopulmonary window for pulmonary atresia/stenosis and major aortopulmonary collaterals. Ann Thorac Surg 94: 842-848. [Crossref]
- Kim H, Sung SC, Choi KH, Lee HD, Ban GH et al. (2015) A central shunt to rehabilitate diminutive pulmonary arteries in patients with pulmonary atresia with ventricular septal defect. J Thorac Cardiovasc Surg 149: 515-520. [Crossref]
- Hibino N, He D, Yuan F, Yu JH, Jonas R (2014) Growth of diminutive central pulmonary arteries after right ventricle to pulmonary artery homograft implantation. Ann Thorac Surg 97: 2129-2133. [Crossref]
- Mumtaz MA, Rosenthal G, Qureshi A, Prieto L, Preminger T et al. (2008) Melbourne shunt promotes growth of diminutive central pulmonary arteries in patients with pulmonary atresia, ventricular septal defect, and systemic-to-pulmonary collateral arteries. Ann Thorac Surg 85: 2079-2083. [Crossref]
- Hofferberth SC, Esch JJ, Zurakowski D, Baird CW, Mayer JE et al. (2018) Pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: collateral vessel disease burden and unifocalisation strategies. Cardiol Young 28: 1091-1098. [Crossref]
- Trezzi M, Albanese SB, Albano A, Rinelli G, D'Anna C et al. (2017) Impact of Pulmonary Flow Study Pressure on Outcomes After One-Stage Unifocalization. Ann Thorac Surg 104: 2080-2086. [Crossref]
- Bauser Heaton H, Borquez A, Asija R, Wise Faberowski L, Zhang Y et al. (2018) Pulmonary reinterventions after complete unifocalization and repair in infants and young children with tetralogy of Fallot with major aortopulmonary collaterals. J Thorac Cardiovasc Surg 155: 1696-1707. [Crossref]
- Bauser Heaton H, Borquez A, Han B, Ladd M, Asija R et al. (2017) Programmatic Approach to Management of Tetralogy of Fallot With Major Aortopulmonary Collateral Arteries: A 15-Year Experience With 458 Patients. Circ Cardiovasc Interv 10: e004952. [Crossref]
- Soquet J, Liava'a M, Eastaugh L, Konstantinov IE, Brink J et al. (2017) Achievements and Limitations of a Strategy of Rehabilitation of Native Pulmonary Vessels in Pulmonary Atresia, Ventricular Septal Defect, and Major Aortopulmonary Collateral Arteries. Ann Thorac Surg 103: 1519-1526. [Crossref]
- Ikeda T, Ikai A (2015) Pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: single-stage complete unifocalization. Multimed Man Cardiothorac Surg. [Crossref]
- Watanabe N, Mainwaring RD, Reddy VM, Palmon M, Hanley FL (2014) Early complete repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Ann Thorac Surg 97: 909-915. [Crossref]
- Mei J, Ding FB, Zhu JQ, Bao CR, Xie X et al. (2010) A novel two-stage complete repair method for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Chin Med J (Engl) 123: 259-264. [Crossref]
- Honjo O, Al Radi OO, MacDonald C, Tran KC, Sapra P et al. (2009) The functional intraoperative pulmonary blood flow study is a more sensitive predictor than preoperative anatomy for right ventricular pressure and physiologic tolerance of ventricular septal defect closure after complete unifocalization in patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collaterals. Circulation 120: S46-S52. [Crossref]
- Song SW, Park HK, Park YH, Cho BK (2009) Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries. Circ J 73: 516-522. [Crossref]
- Ishibashi N, Shin'oka T, Ishiyama M, Sakamoto T, Kurosawa H (2007) Clinical results of staged repair with complete unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Eur J Cardiothorac Surg 32: 202-208. [Crossref]
- Amark KM, Karamlou T, O'Carroll A, MacDonald C, Freedom RM et al. (2006) Independent factors associated with mortality, reintervention, and achievement of complete repair in children with pulmonary atresia with ventricular septal defect. J Am Coll Cardiol 47: 1448-1456. [Crossref]
- Griselli M, McGuirk SP, Winlaw DS, Stümper O, de Giovanni JV et al. (2004) The influence of pulmonary artery morphology on the results of operations for major aortopulmonary collateral arteries and complex congenital heart defects. J Thorac Cardiovasc Surg 127: 251-258. [Crossref]
- Duncan BW, Mee RB, Prieto LR, Rosenthal GL, Mesia CI et al. (2003) Staged repair of tetralogy of Fallot with pulmonary atresia and major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg 126: 694-702. [Crossref]
- Gupta A, Odim J, Levi D, Chang RK, Laks H (2003) Staged repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: experience with 104 patients. J Thorac Cardiovasc Surg 126: 1746-1752. [Crossref]
- Carotti A, Di Donato RM, Squitieri C, Guccione P, Catena G (1998) Total repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: an integrated approach. J Thorac Cardiovasc Surg 116: 914-923. [Crossref]
