Journals
Mini-invasive treatment of median arcuate ligament syndrome, a case report
A B S T R A C T
Introduction: Median arcuate ligament syndrome (MALS), also known as Dunbar syndrome or celiac artery compression syndrome, is a rare disorder due to external compression of the celiac trunk (CT) by the median arcuate ligament (MAL). The diagnosis is difficult and often one of exclusion, because of its nonspecific symptoms that overlap with other forms of chronic intestinal ischemia. Laparoscopic approach is considered to be an optimal therapeutic option.
Material and Surgical Techniques: We describe the case of a 40-year-old woman who presented with a 4 years-long clinical history of postprandial abdominal pain, occasional vomiting and severe weight loss in the last year. An abdominal CT scan demonstrated an external compression of the CT and the patient underwent laparoscopic decompression by division of the MAL. Postoperative course was uneventful and the patient was discharged on the 5th postoperative day.
Discussion: Despite being MALS a rare disease, it must be kept in the differential diagnosis of abdominal pain. Diagnosis is difficult and often requires 2nd level investigations. Laparoscopy can be useful both as a diagnostic and curative approach. The laparoscopic division of the MAL is a feasible and safe procedure, leading to an improved quality of life.
Keywords
Median arcuat ligament, Dunbar syndrome, celiac trunk, abdominal pain, laparoscopy.
Introduction
Median arcuate ligament syndrome (MALS), also known as Dunbar syndrome, refers to chronic and recurrent abdominal pain resulting from the compression of the celiac trunk (CT) by the median arcuate ligament (MAL). The syndrome was first described by Harjola in 1963 and Dunbar in 1965. The incidence of CT compression by MAL at autoptic findings is up to one-third [1]. However, clinical symptoms attributable to MALS appear only in 4-7% of patients presenting with CT compression on imaging studies [2-3]. Dunbar syndrome is more prevalent in women (4:1) between 40-60 years of age with a thin body habitus. The diagnosis is difficult and often rendered by excluding differential diagnoses of intermittent abdominal pain. Laparoscopic approach is considered to be the most effective and less invasive, both in the diagnostic workup and in the treatment, consisting in the division of MAL. The mini-invasive is feasible by a laparoscopic surgeon with vascular skills because of increased risk of injury of the abdominal aorta, in comparison to open approach.
Case Report
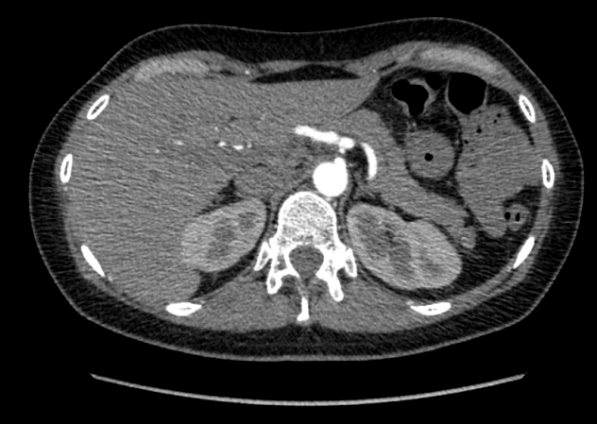
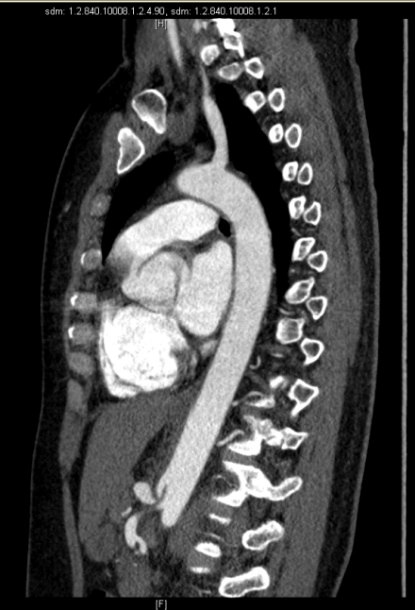
We report the case of a 40-year-old Caucasian woman, who presented to our attention after a 4 years-long history of moderate postprandial epigastric pain, associated with occasionally nausea, vomiting and dyspepsia. The patient reported an unintentional weight loss of 15 Kg in the last year. She had no history of chronic disease or drug use and was a non-smoker. Her family history was negative for neoplasm and her only past surgery was a laparoscopic appendectomy. Her physical examination was mostly normal, except for a mild epigastric tenderness on palpation. The results of laboratory tests, abdominal ultrasound and gastroscopy were also within normal limits. Because of the persistence of symptoms despite therapy with prokinetic drugs, we performed an abdominal CT-angiogram that revealed the presence of a proximal stenosis of the CT, with post stenotic dilatation. The finding was suggestive of compression by median arcuate ligament.
Figure 1-2: Axial and sagittal view in CT scan that revealed proximal stenosis of the celiac trunk with post stenotic dilatation.
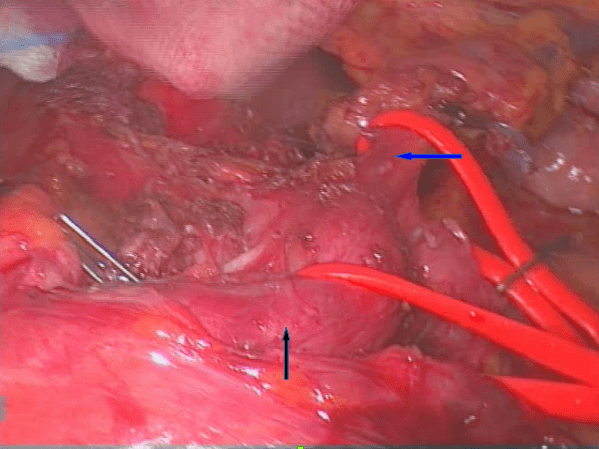
We proposed to the patient a laparoscopic exploration and section of the MAL and obtained the informed consent. Surgical exploration was performed under general anaesthesia, whit the patient in supine position with open legs and the first surgeon between the patient’s legs. Pneumoperitoneum was established with Palmer’s point Veress needle technique. A camera port was inserted periumbilical and then 4 more trocars were placed in the following positions: two in the left subcostal area, 1 in the right subcostal area and 1 in the epigastrium (under the xyphoid, in order to elevate the left lobe of the liver). Following abdominal exploration, the gastrohepatic ligament was transected in order to expose and dissect the crura. The esophagus was pulled up with a tape and the anterior surface of the abdominal aorta was exposed, showing a compression on CT by firm adhesions. The Left Gastric Artery (LGA) and the Common Hepatic Artery (CHA) were isolated and hold medially and laterally with a tape. Following this manoeuvre, the fibrotic and muscular structures imprinting the origin of the CT were well visualized and then divided by using an ultrasonic energy device. The arterial flow in the branches of the celiac trunk was proved to be optimal at the end of surgery. A drain was placed near the aorta and removed on the 2nd postoperative day. The postoperative course was uneventful: the patient progressed to soft foods on postoperative day 1 and was discharged on day 5. Three months after her discharge, the patient reported that she was pain-free and that she would eat regularly without any discomfort.
Figure 3: The left gastric artery (blue arrow) and Common Hepatic Artery (black arrow) were hold medially and laterally with tape to visualize the the fibromuscular structures that imprinting the origin of the Celiac Trunk.
Discussion
Dunbar syndrome or median arcuate ligament syndrome (MALS) is a rare and controversial vascular compression syndrome caused by MAL; its etiology and pathophysiology are still not fully understood. The anatomy of the celiac region was described long before the relationship between abdominal symptoms and compression of the CT was suggested. The esophageal hiatus is separated from the aortic hiatus by fusion of the arms of the left and right crura. The MAL appear as a fibrous arch passing over the aorta at the level of L1 just above the origin of the CT. The celiac ganglia lie just below and lateral to the CT in intimate juxtaposition. In 16% of patients a low MAL covers the CT and can compress it. In 1971, Linder et al. offered a detailed description of the anatomy of the celiac region. They found that the relationship between the CT and the MAL is extremely variable, this being due to multiple factors such as the height and the angle of origin of the CT, the height of the MAL, the habitus, age and sex of the patient and the thickness and tenacity of the celiac ganglia and plexus. For example, in their studies revealed that the ectomorphic females have a greater incidence of MALS, because have a closer relationship between the two structures with a higher origin of the CT and a lower MAL (the ratio is 4:1 for women, between the ages of 40 and 60 years). Asymptomatic compression or stenosis of the CT is common, probably due to hemodynamic compensation mechanisms: autopsy studies have shown that the CT is compressed by the MAL in up to one-third of individuals [1]. Vascular and neurologic theories have been suggested to elucidate the symptomatic cases of MALS [4]. Compression of the CT by MAL causes mesenteric ischemia, with abdominal angina if the arterial blood flow is reduced from 60-75%, because there is an extensive collateral blood supply to the mesenterium from other blood vessels (superior and inferior mesenteric artery). The range of stenosis of the CT can vary sensitively during the respiratory phase and its clinical impact depends on the blood request of the abdominal organs). In fact, the position of CT and MAL changes during the respiratory cycle. Lee et al., proved through MR angiography that the aorta and its branches move cranially during expiration, resulting in the MAL moving over the CT to cause maximal compression on expiration [5]. Other authors emphasize the role of the celiac plexus and the celiac ganglion by applying an external compression on the CT or by causing a splanchnic vasoconstriction due to neurologic stimulation [6]. In this theory, pain relief after CT decompression results not from improvement in postprandial flow but from destruction of the splanchnic nerves during the surgical exposure of the artery. MALS may be suspected in middle-aged (40 to 60 years) female patients based upon a clinical trial of postprandial epigastric pain (reported by 94% of the patients), nausea or vomiting and weight loss. Physical examination is usually normal: underweight may be evident and epigastric tenderness may be present, but these clinical signs are not specific of the syndrome. The diagnosis requires angiography to confirm the vascular compression. The gold standard in the diagnosis is the selective arteriography which should be performed during both inspiration and expiration, although the first approach is often with an abdominal CT-scan or MR [7]. In our case, we made diagnosis with an abdominal CT-angiogram, showing the following suggestive findings: proximal stenosis of the CT at the origin and post stenotic dilatation. It is undeniable that the diagnostic path is very difficult in the most cases, with an average duration of symptoms of 34 months before a diagnosis is obtained. The implementation of multi-slice CT and the CT-angiography allows the acquisition of thinner images with increased resolution. Catheter-based arteriography is rarely needed to confirm the diagnosis. Doppler US has been reported to have a high sensitivity for the diagnosis of MALS: ultrasound may show an elevated systolic velocity at the origin of the CT during expiration and normal velocities in inspiration. Other physiologic tests can help identify MALS, but they are rarely used in clinical practice. The treatment of symptomatic MALS aims to restore celiac blood flow by relieving the extrinsic compression on the vessel. Angioplasty and stenting of CT have been described as reasonable tool in the treatment of MALS, but we believe that the treatment is primarily a surgical one. First Dunbar in 1965 Dunbar and then Harjola in 1967 reported their experience in surgical treatments of MAL with excellent results. In 2000, the first laparoscopic decompression was published by Roayaie et al. [8]. Both open and minimally invasive approach can be used, but we suggest laparoscopy (standard or robotic-assisted) because of its well-known advantages: smaller incisions, improved view of the surgical field, decreased pain and postoperative morbidity and shorter recovery time [9]. Exposure of the aorto-celiac axis might be considered as the most challenging part of the surgical procedure. Confirmation of the total release of celiac trunk can be obtained by arteriography or intraoperative doppler ultrasound. In our case, we considered the visual inspection of an increased celiac flow to be sufficient. The rate of open conversion in Literature ranges between 13% and 27% of cases, due to either inadequate visualization of the anatomy or excessive bleeding. When appropriately selected, the majority of patients had immediate pain relief (78-96%) after laparoscopic decompression. In case of recurrence of symptoms, patients are qualified for percutaneous angioplasty and stent implantation [10].
Conclusion
MALS is a rare condition that should be considered as a differential diagnosis in patients with nonspecific postprandial abdominal pain, particularly in middle-aged females. The diagnosis is difficult, and a laparoscopic exploration is sometimes necessary for confirmation. Laparoscopic treatment is a feasible and safe approach that can lead to an immediate resolution of symptoms and an improved quality of life.
Article Info
Article Type
Case ReportPublication history
Received: Sat 09, Mar 2019Accepted: Thu 25, Apr 2019
Published: Thu 23, May 2019
Copyright
© 2023 Marcodomenico Mazza. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.02.012
Author Info
Angelica Ganss Angelo Marzullo Irene Spilimbergo Marcodomenico Mazza Mariangela Ruperto Marzia Riccardo Maurizio Balduino Maurizio De Luca Nicola Passuello
Corresponding Author
Marcodomenico MazzaDepartment of General Surgery, S. Giacomo Hospital, Castelfranco Veneto (Treviso), Italy
Figures & Tables
References
- Linder HH, Kemprud E (1971) A clinicoanatomical study of the arcuate ligament of the diaphragm. Arch Surg 103: 600-605. [Crossref]
- Park CM, Chung JW, Kim HB, Shin SJ, Park JH (2001) Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol 2: 8-13. [Crossref]
- Kazan V, Qu W, Al-Natour M, Abbas J, Nazzal M (2013) Celiac artery compression syndrome: a radiological finding without clinical symptoms? Vascular 21: 293-299. [Crossref]
- Baskan O, Kaya E, Gungoren FZ, Erol C (2015) Compression of the celiac artery by the median arcuate ligament: multidetector computed tomography findings and characteristics. Can Assoc Radiol 66: 272-276. [Crossref]
- Lee VS, Morgan JN, Tan AG, Pandharipande PV, Krinsky GA et al. (2003) Celiac artery compression by the median arcuate ligament: a pitfall of end-expiratory MR imaging. Radiology 228: 437-442. [Crossref]
- Dunbar JD, Molnar W, Beman FF, Marable SA (1965) Compression of celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 95: 731-744. [Crossref]
- Geelkerken RH, Van Bockel JH (1995) Mesenteric vascular disease: a review of diagnostic methods and therapies. Cardiovasc Surg 3: 247-260.
- Roayaie S, Jossart G, Gitlitz D, Lamparello P, Hollier L et al. (2000) Laparoscopic release of celiac artery compression syndrome facilitated by laparoscopic ultrasound scanning to confirm restoration offlow. J Vasc Surg 32: 814-817. [Crossref]
- Jimenez JC, Harlander-Locke M, Dutson EP (2012) Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg 56: 869-873. [Crossref]
- Maciej Michalik, Natalia Dowgiallo-Wnukiewicz, Pawel Lech , Kaja Majda, Piotr Gutowski et al. (2016) Hybrid (laparoscopy + stent) treatment of celiac trunk compression syndrome (Dunbar syndrome, median arcuate ligament syndrome (MALS). Wideochir Inne Tech Maloinwazyjne 11: 236-239. [Crossref]
