Most Appropriate Radiation Therapy Techniques for The Breast Cancer Treatment: Dosimetric Analysis of Three Different Radiation Therapy Methods
A B S T R A C T
Background: Radiation-induced cardiac events and pneumonitis are the major late radiotherapy side effects.
Methods: We explored the differences in dose distribution between 3D CRT, IMRT and VMAT in 125 women with unilateral breast cancer. The various dose distribution to the tumor and OARs were studied for left and right sided irradiation.
Results: Upon using 3D CRT breasts with lymph nodes, we observed a significant dose increase in the ipsilateral lung. Having irradiated only the right breast, there was a negligible Dmean difference to the adjacent lung between three methods, likewise for the myocardium in left breast cases. For right-sided cases 3D CRT showed the lowest doses in myocardium.
Conclusion: Radiation oncologists should consider VMAT if conditions do not allow devising three plans with different methods. This in turn will reduce cardio and pneumotoxicity of left breast cancer treatment. In the treatment of right breast cancer, physicians should focus on 3D CRT.
Keywords
Radiotherapy, breast cancer, 3D CRT, VMAT, IMRT, personal treatment planning
Introduction
According to the World Health Organization, in 2020 breast cancer were diagnosed in 2.3 million women and 685,000 deaths globally. It makes this type of cancer the most globally prevalent among women [1]. The incidence of breast cancer is widespread throughout the world and disability-adjusted life years (DALYs) in women with breast cancer higher than in women with other cancer type. Surgery and radiation therapy (RT) plays a key role in the treatment of breast cancer [2]. RT may be used to treat breast cancer at almost every stage [3]. It is an effective way to reduce risk of breast cancer recurrence after surgery [4-6]. In addition, RT is commonly used to ease the symptoms caused by cancer that has spread to other parts of the body (metastatic breast cancer) [7, 8]. However, RT, as well as surgery and chemotherapy, has both curative effects and undesirable complications. Physicians are most concerned about cardiac complications and radiation-induced secondary malignancies [9-11]. These complications can negate success in defeating breast cancer. The cure/complication ratio may depend on the adequacy of RT techniques.
We searched for the initial 50 articles in the PubMed search engine that appeared in the “breast cancer radiotherapy” category, namely 25 each in the “best match” option and the “more recent” option, directly related to RT techniques. Eight articles mentioned that the most common methods used for breast cancer radiotherapy from 2015 to 2022 were 3-dimensional conformal radiotherapy (3D CRT), intensity-modulated RT (IMRT), and volumetric modulated arc therapy (VMAT) [12-19]. With a pronounced variety of irradiated volumes in breast cancer depending on the disease stage and surgical approach, there is a lack of consensus on the appropriate method of irradiation which has been predominantly used or avoided. At present, a personalized approach to treatment has gained attention taking into account all the anatomical features of each patient.
To the best of our knowledge, the clinical and dosimetry analysis of 3D CRT, IMRT and VMAT for different stages and sides of breast cancer disease has not been studied. This study aimed at assessing the most appropriate one. Subsequently, we set the following objectives: i) to study and compare the clinical and dosimetric characteristics of three methods of radiation treatment for breast cancer, namely 3D CRT, IMRT and VMAT; ii) to identify differences in the ionizing radiation doses distribution in the planned target volume (PTV) and in organs at risk OARs (Contralateral breast, right coronary artery (RCA), left anterior descending artery (LADA), myocardium, contralateral and ipsilateral lung) in radiation treatment of the right and left breasts cancer separately for different stages of the disease.
Materials and Methods
I Patient Characteristics
The study included 125 women with unilateral breast cancer stages from 0 (pTisN0M0) to IIIC (pT1-4 N1-3 M0) who underwent adjuvant radiation treatment at the Department of Radiation Oncology from 2015 to 2020. 66 (53%) and 59 (47%) patients of the primary group had cancer of the left and right breasts, respectively. The median age was 53 years. Patients with stages I-III accounted for 93% of the samples. The disease stage was determined according to the international TNM system of the 7th revision of 2010. All patients had resection with no gross or microscopic tumor remains (R0). The prescribed radiation therapy dose was 50 Gy in 25 fractions. We planned the radiation only to the PTV.
II Target Volume Delineation
Radiotherapy simulation was performed on a Philips Brilliance Big Bore spiral computed tomography (CT) scanner, Koninklijke Philips N.V (Amsterdam, Netherlands) without contrast enhancement, using Qfix (Avondale, USA), a QUEST™ Breastboard RT-4543 fixation device. The breast board tilt was set to 5º for all patients. The arms were raised above the head at an angle of approximately 120º to the median axis of the body. CT scans were performed with free breathing (FB) on the area between the lower jawbone and the diaphragm. Each patient was instructed to breathe smoothly and evenly during the CT simulation and subsequent treatment sessions. The chest movement was tracked during the CT simulation and the maximum difference in movement was 1.5 - 2 mm. We took into account this difference on PTV and used pseudo-skin flash method with virtual bolus. CT slices were 2 mm thick. All patients received radiation treatment on TrueBeam or trilogy linear accelerators with millenium 120-multileaf collimator (MLC) manufactured by Varian Medical System (Palo Alto, USA). We performed radiation therapy planning and subsequent radiation treatment with a treatment plan developed by a medical physicist and radiation oncologist. For research tasks, the remaining exposure methods/dosimetry plans were simulated in the treatment planning system.
The clinical target volume (CTV) following breast-conserving surgery (BCS) included the entire breast parenchyma visible on CT images. Depending on the histological characteristics of the tumor, disease stage, operation, and individual characteristics, the CTV could include the lymph nodes of I-IV levels, parasternal lymph nodes, and the superficial tissues of the chest wall up to the ribs.
III Treatment Planning
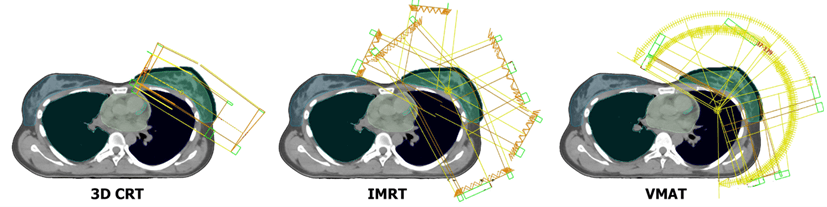
The 3D CRT typically had from 2 to 5 coplanar 6 MV or 10 MV photon beams with a homogeneous fluence, formed by a MLC. “Field-in-field” method and wedges were used in planning if necessary. 10 MV energy was used when the PTV volume exceeded 3000 cc and for upper-level lymph nodes if necessary. The IMRT plans had from 5 to 7 coplanar fields with 6 MV or 10 MV photon energy equally distributed around tangential field angles (gantry angles between 179-300° for left and 181-60° for right side respectively). The VMAT typically was performed by creating 3-7 noncoplanar arcs of gantry rotation on the side of the irradiated breast. Photon energies were also 6 MV or 10 MV. We selected the values of the angles of the beginning and end of the arc ranging from 179° to 310° for left-side and 181° to 50° for right-sided irradiations. When more than 3 arcs were used, each arc was split in two to have a better avoidance of OAR (mainly heart and lung) and one of them had a couch rotation of 13° or 347°. Figure 1 shows an example of fields positioning for 3D CRT, IMRT and VMAT techniques.
Figure 1: An example of fields positioning for 3D CRT, IMRT and VMAT treatment plans.
Prescriptions for the VMAT and IMRT plans were: 100% dose absorbed in 95% of the PTV and 95% dose absorbed in 98% of the PTV, and maximum dose (Dmax = D 0.03cc (dose to 0.03cc volume) was 107 % to 110%. We relaxed the plan acceptance criteria as follows when these values were not achievable: 100% dose for 90% PTV and 95% dose for 95% PTV and Dmax < 120%. Dmax was considered as 0.03 cc. The plan acceptance criteria for 3D CRT was: 95% dose absorbed in 95% PTV, 100% dose in 90% PTV, Dmax between 107 % to 110%. For unachievable prescription, we used the following criteria: 95% dose in 90% PTV, 90% dose in 95% PTV, and Dmax < 120%.
We developed irradiation plans in the eclipse 15.5 planning treatment system manufactured by the Varian Medical System (Palo Alto, USA). Limitations for the critical organs included parameters from the RTOG 1005 protocol [20]. Following constraints were used: for myocardium for left-sided breast V20Gy < 5%, V10Gy < 30%, Dmean < 5 Gy with acceptable variations V25Gy < 5%, V10Gy < 35% and Dmean < 5 Gy, for right-sided breast V20Gy < 0%, V10Gy < 10%, Dmean < 4 Gy with acceptable variations V25Gy < 0%, V10Gy < 15% and Dmean < 5 Gy; for ipsilateral lung - V20Gy < 15%, V10Gy < 35%, V5Gy < 50%, Dmean < 12 Gy with acceptable variations V20Gy < 20%, V10Gy < 40% and V5Gy < 60% and Dmean < 15 Gy; for contralateral lung it were V5Gy < 10%, Dmean < 3 Gy with acceptable variations V5Gy < 20% and Dmean < 4 Gy; for contralateral breast it was Dmean < 5 Gy with acceptable variation Dmean < 6 Gy.
In all patients, the therapeutic placement was verified on the treatment couch by combining the image obtained in the cone beam CT (CBCT) of the linear accelerator with CT-simulation image by RT technician and radiation oncologist. All treatment plans were created taking into account the implementation of all prescribed constraints. With VMAT 76% patients achieved treatment constraints, with IMRT - 70% patients and with 3D CRT - 66% patients.
IV Plan Evaluation and Statistical Analysis
The treatment plans were visually analysed by dose distribution and dose/volume histogram in TPS. We used identical PTV contours and critical volumes for three treatment methods in one patient. We conducted data analysis using the non-parametric Kruskal-Wallis test for multiple samples, considering the localization of the process (right or left side), the configuration of the PTV (with or without lymph nodes), and irradiation methods used (3D CRT, IMRT or VMAT). The Tukey’s honest significance difference (HSD) test was used for paired group comparison. All statistical tests were two-tailed. P-values < 0.05 denoted statistical significance.
For a comparative assessment, all demonstration sections were equally considered, such as transverse at the level of the eighth thoracic vertebra (the level at the beginning of the left coronary artery), frontal at the middle of the anterior-posterior body size, and sagittal at the level of the midclavicular line on the side of the disease.
Results
By summing up the prescribed dose in the PTV, the 3D CRT, IMRT and VMAT methods in modern technical and computational performance (on TrueBeam or Trilogy linear accelerators according to the plans calculated in the Eclipse v.15.5 Varian Medical System (Palo Alto, USA)) could deliver at least 95% of the absorbed dose to at least 95% of the PTV, with the significant differences in the distribution of absorbed doses outside the PTV and in individual OARs. Table 1 summarizes data on the average Dmean (SD) in the OARs of 3D CRT, IMRT and VMAT depending on the PTV for right- or left-side irradiation. Table 2 summarizes data on P-values of 3D CRT, IMRT and VMAT.
Table 1: Average dose distribution (Dmean)
and standard deviation (SD) in the OARs of 3D CRT, IMRT and VMAT depending on
the treatment volume.
|
|
|
Organ
at risk |
|||||||||||
|
|
|
Contralateral
Breast |
RCA |
LADA |
Myocardium |
Contralateral
Lung |
Ipsilateral
Lung |
||||||
|
Treatment
Volume |
Technique |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
|
Right
Breast with lymphnodes |
3D
CRT |
0,94 |
0,59 |
4,20 |
3,56 |
0,55 |
0,24 |
1,23 |
0,36 |
0,36 |
0,17 |
17,71 |
2,98 |
|
IMRT |
2,82 |
1,23 |
9,99 |
5,74 |
4,44 |
3,42 |
5,23 |
2,25 |
2,54 |
1,10 |
14,15 |
2,39 |
|
|
VMAT |
4,06 |
0,99 |
10,26 |
4,56 |
6,22 |
2,80 |
5,22 |
2,17 |
3,56 |
0,88 |
10,83 |
1,99 |
|
|
Left
Breast with lymphnodes |
3D
CRT |
0,80 |
0,77 |
3,20 |
2,88 |
19,72 |
13,73 |
7,00 |
3,95 |
0,41 |
0,34 |
16,29 |
4,63 |
|
IMRT |
2,78 |
1,43 |
7,29 |
3,59 |
12,32 |
4,88 |
7,50 |
2,80 |
2,87 |
1,23 |
12,93 |
2,21 |
|
|
VMAT |
3,72 |
1,42 |
9,66 |
5,41 |
9,33 |
4,61 |
6,10 |
2,33 |
3,53 |
1,00 |
9,83 |
1,62 |
|
|
Right
Breast |
3D
CRT |
0,26 |
0,26 |
1,47 |
0,39 |
0,18 |
0,14 |
0,54 |
0,15 |
0,09 |
0,08 |
11,24 |
4,67 |
|
IMRT |
1,36 |
0,79 |
3,88 |
2,00 |
1,48 |
1,03 |
2,41 |
1,32 |
1,23 |
0,73 |
9,83 |
2,81 |
|
|
VMAT |
2,85 |
0,99 |
8,19 |
3,11 |
4,72 |
1,51 |
5,25 |
1,57 |
2,71 |
0,88 |
9,72 |
1,94 |
|
|
Left
Breast |
3D
CRT |
0,92 |
0,77 |
1,77 |
0,88 |
14,23 |
9,89 |
6,10 |
3,83 |
0,25 |
0,17 |
11,20 |
3,53 |
|
IMRT |
2,38 |
1,40 |
3,92 |
1,34 |
9,32 |
5,08 |
5,50 |
2,08 |
1,77 |
0,64 |
9,70 |
1,78 |
|
|
VMAT |
3,17 |
1,04 |
5,92 |
2,78 |
6,99 |
3,68 |
5,39 |
2,31 |
2,98 |
0,85 |
8,71 |
1,33 |
|
Table 2: P-values of 3D CRT, IMRT and VMAT multiple
(Kruskal-Wallis test) and paired group (Tukey’s HSD test) comparison depending
on the treatment volume.
|
Organ
at risk |
|||||||
|
Treatment
Volume |
p-value |
Contralateral
Breast |
RCA |
LADA |
Myocardium |
Contralateral
Lung |
Ipsilateral
Lung |
|
Right
Breast with lymphnodes |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
3D
vs IMRT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
IMRT
vs VMAT |
<0.001 |
0,978 |
0,037 |
0,995 |
<0.001 |
<0.001 |
|
|
Left
Breast with lymphnodes |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
0,006 |
0,176 |
<0.001 |
<0.001 |
|
3D
vs IMRT |
<0.001 |
0,052 |
0,003 |
0,697 |
<0.001 |
<0.001 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
<0.001 |
0,676 |
<0.001 |
<0.001 |
|
|
IMRT
vs VMAT |
0,006 |
0,042 |
0,41 |
0,227 |
0,006 |
<0.001 |
|
|
Right
Breast |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
0,718 |
|
3D
vs IMRT |
0,001 |
0,068 |
0,022 |
0,013 |
<0.001 |
0,625 |
|
|
3D
vs VMAT |
<0.001 |
0,001 |
<0.001 |
<0.001 |
<0.001 |
0,55 |
|
|
IMRT
vs VMAT |
<0.001 |
0,262 |
<0.001 |
0,009 |
<0.001 |
0,992 |
|
|
Left
Breast |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
0,245 |
0,897 |
<0.001 |
0,187 |
|
3D
vs IMRT |
0,004 |
0,039 |
0,254 |
0,885 |
<0.001 |
0,355 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
0,06 |
0,842 |
<0.001 |
0,07 |
|
|
IMRT
vs VMAT |
0,414 |
0,057 |
0,723 |
0,995 |
<0.001 |
0,634 |
|
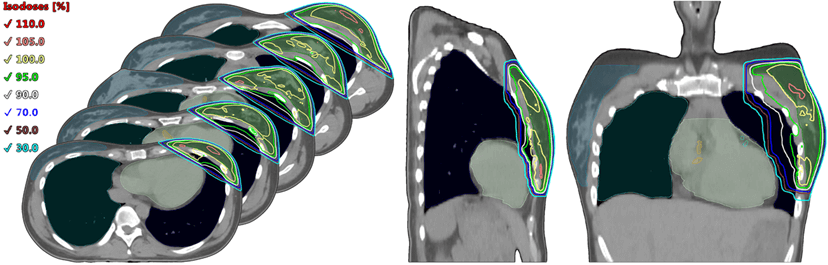
When using 3D CRT, in comparison with IMRT and VMAT, with any irradiation volume on both the right and left, an increase in the high dose (50-90%) on the ipsilateral lung in its segment adjacent to the dorso-medial side of the target, which is 10-15% of the lung volume, was observed. The contralateral organs with 3D CRT received the lowest dose compared with VMAT and IMRT. Especially, the lowest dose load on the contralateral lung was obtained with 3D CRT for all cases (0.41-0.09 ± 0.34-0.08 Gy), while with IMRT it was 2.87-1.23 ± 1.23-0.73 Gy and with VMAT - 3.56-2.71 ± 0.88 Gy (p<0.001). Figure 2 depicts typical dose distribution for 3D CRT technique for left breast.
Figure 2: An example of dose distribution by 3D CRT technique.
While irradiating the left breast with lymph nodes with 3D CRT, the absorbed dose in LADA (19.72 ± 13.73 Gy) is significantly higher than that with VMAT which was 9.33 ± 4.61 Gy (p<0.001), and without lymph nodes not significant difference was observed - 14.23 ± 9.89 Gy for 3D CRT and 6.99 ± 3.68 Gy for VMAT. This difference can be reduced only by treating patients with deep inspiration breath-hold (DIBH). Otherwise, VMAT allows significant dose reduction for LADA at any amount of volume of radiation on the left. However, further discussion of this is beyond the scope of this study.
The LADA with left-sided irradiation received the highest dose for any irradiation method, compared with the right-sided one since the part of the lung is displaced by that part of the heart where the LADA lies. The Dmean difference in absorbed dose with right-sided irradiation with lymph nodes between the methods were: 3D CRT (0.55 ± 0.24) - VMAT (6.22 ± 2.80), (p<0.001); without lymph nodes was: 3D CRT (0.18 ± 0.14) - VMAT (4.72 ± 1.51), (p<0.001) respectively. The RCA received a dose in the majority of cases from a Low Dose Bath (LDB), and its level was highest upon using VMAT (10.26-5.92 ± 4.56-2.78 Gy), by Dmean two to four times, compared with 3D CRT (4.20-1.47 ± 3.56-0.39 Gy) (p≤0.001 in pairwise comparison respectively) regardless of the irradiation side.
IMRT decreased the load on the RCA by 25% to 53%, compared with VMAT except right breast with lymph nodes cases where it has similar value. The statistically significant difference in the RCA Dmean between VMAT and IMRT was observed only for the left side breast with and without lymph nodes (p=0.042 and 0.057 respectively). An example of IMRT dose distribution is shown in (Figure 3). When only the right breast irradiated up to 50 Gy, Dmean to the adjacent lung with 3D CRT was 11.24 ± 4.67 Gy, with IMRT - 9.83 ± 2.81 Gy, and with VMAT - 9.72 ± 1.94 Gy (p=0.718); the dose to other OARs with 3D CRT is significantly lower than with IMRT-VMAT. The only disadvantage of 3D CRT method is that it irradiates approximately 10% to 15% of the volume of the adjacent lung with a 80% dose. Thus, clinicians should prefer 3D CRT in cases of cancer of the right breast, without the need to irradiate the regional lymph nodes area.
Figure 3: An example of dose distribution by IMRT technique.
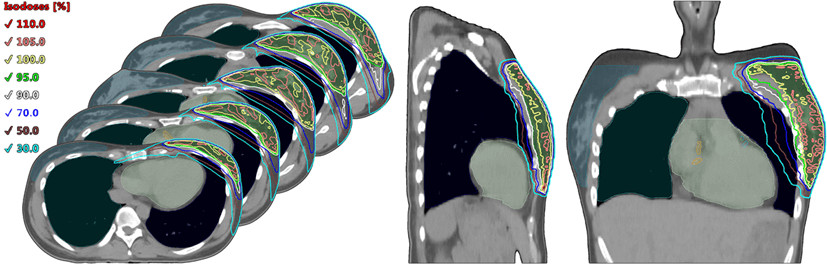
For breasts with lymph nodes regardless of the side VMAT showed the lowest dose to ipsilateral lung between techniques (10.83-9.83 ± 1.99-1.62 Gy), when with 3D CRT method it was 17.71-16.29 ± 2.98-4.63 Gy and with IMRT - 14.15-12.93 ± 2.39-2.21 Gy (p<0.001). This dose reduction for VMAT appears due to high gradients in OAR created during the plan optimization, specifically for high dose levels more than 50%. That comes along with LDB increase, while for 3D and IMRT methods it is a vice versa. An example of VMAT dose distribution is shown in (Figure 4).
Figure 4: An example of dose distribution by VMAT technique.
For the myocardium in left breast cases with or without lymph nodes there was not significant difference between the methods. With 3D CRT (7.00-6.10 ± 3.95-3.83 Gy), IMRT (7.50-5.50 ± 2.80-2.08 Gy) and with VMAT (6.10-5.39 ± 2.33-2.31 Gy) mean doses was observed (p>0.05). When for right-sided cases 3D CRT showed the lowest doses in myocardium (1.23-0.54 ± 0.36-0.15 Gy) in comparison with IMRT (5.23-2.41 ± 2.25-1.32 Gy) and VMAT methods (5.22-5.25 ± 2.17-1.57 Gy) (p<0.001). This dose increases for right breast in myocardium with VMAT and IMRT techniques arises from LDB inherent to them.
Discussion and Conclusion
Despite advances in radiation therapy techniques, the toxicities from RT in patients with breast cancer still cause concern, including cardiac/pneumo toxicities as well as the most common side effect of RT in breast cancer - radiation dermatitis and the most dangerous - second malignancies [9, 21]. Сonsidering the benefits of RT in the form of prolong survival and reduce the frequency of relapses, radiation-induced side effects should be considered as well. Yasser Abo-Madyan et al. estimated the risks for developing a solid second cancer after radiotherapy of breast cancer. They found that the second cancer risk after 3D CRT or IMRT is lower than for VMAT by about 34% for the linear model and 50% for the linear-exponential and plateau models, respectively [22]. Compared with non-irradiated patients, patients who have been irradiated for breast cancer have a significantly increased risk of cardiac mortality. Numbers of patients with radiation-induced heart disease in breast cancer have increased, and most of them have ischaemic heart disease [23]. The absolute radiation-related risk of a major coronary event also increases significantly in breast cancer patients with preexisting cardiac risk factors. To lower the radiation toxicity and reduce the risk of radiation-induced cancer, selecting a reasonable RT modality plays a critical role.
Liu H. et al. compared dosimetric differences based on three types of radiotherapy plans for postoperative left breast cancer only. The authors concluded that IMRT and VMAT plans have a better conformity, than 3D CRT [24]. Ma C. et al. also studied three different radiotherapy techniques for left side breast cancer. Their conclusions were: 5 field- IMRT is more dosimetrically advantageous, compared with field-in-field 3D CRT as well as enhanced heart and left lung sparing and similar PTV coverage compared with two partial arc VMAT [25]. We did not find studies comparing the three irradiation techniques for right-sided breast cancer, as well as studies taking into account different radiation volumes. Das Majumdar S.K. et al., in their study concluded that VMAT and IMRT fared better than the 3D CRT as far dosimetry of high dose volumes was considered. Inverse planning methods that worsened performance were the low dose irradiation of the heart, lung, contralateral breast, and integral dose to the body [26]. In another similar study, Ahmad A. et al. compared VMAT, IMRT and 3D CRT with a one-week hypofractionated radiotherapy regime (26 Gy in 5 fractions) for adjuvant breast radiotherapy. Authors concluded that IMRT and VMAT techniques are feasible and can achieve better dosimetric goals for target and OARs though minimizing the area achieving low dose remains to be a dosimetric concern for VMAT [27].
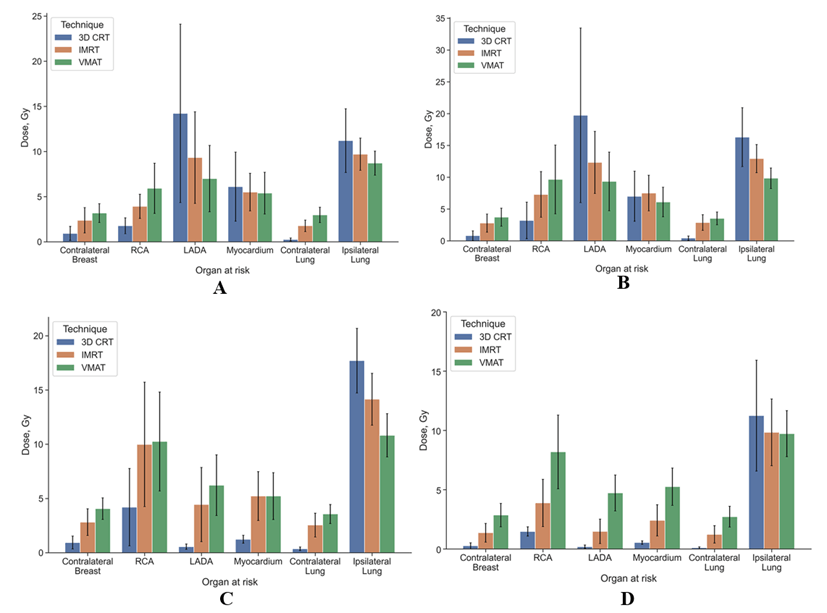
Figure 5: Mean dose with SD in the OARs of 3D CRT, IMRT and VMAT technique for left-side breast cancer with A) lymph nodes and B) without lymph nodes, for right-side breast cancer C)with lymph nodes and D) without lymph nodes.
Recently, many researchers prefer hybrid methods of radiation therapy for breast cancer treatment, such as hybrid- IMRT (H-IMRT) and H-VIMAT to avoid the disadvantages of each method. Mayo C. et al. in 2005 evaluated H-IMRT technique for breast cancer treatment. The 6-field hybrid technique creates the most conformal dose distribution at the expense of more normal tissue receiving low dose [28]. Then in 2015, Lin J et al. analysed the dosimetric performance of 3 treatment techniques: H-VMAT, pure-VMAT, and fixed-field IMRT (F-IMRT) for left-sided breast irradiation. The H-VMAT plan is feasible for whole-breast irradiation of left-sided early breast cancers. Compared with the single model of the F-IMRT plan or the pure-VMAT plan, the H-VMAT plan provides better dose conformity and homogeneity, delivers fewer doses to the ipsilateral lung and the heart, and delivers doses more efficiently [29]. Chen Y. et al. concluded that H-VMAT plans are especially superior to the hybrid IMRT plans with regard to heart dose and treatment delivery time [30]. In another study, Smith S. et al. came to a similar conclusion [31].
In our study, we concluded that radiation oncologists should consider VMAT if conditions do not allow devising three plans with different methods and selecting the best one. This in turn will reduce cardio and pneumotoxicity in the treatment of left breast cancer when included in the PTV of the breast with lymph nodes levels I-IV or without them. In the treatment of right breast cancer, physicians should focus on the use of 3D CRT, particularly in patients younger than 40. The Dmean in the OARs of 3D CRT, IMRT and VMAT technique for both sides breast cancer with lymph nodes and without lymph nodes represented in (Figure 5).
If the clinical equipment enables planning with all three technologies, doctors should know the primary differences that may affect selection. Exposure to IMRT is inevitably associated with an increase in the number of monitor units (MU) and exposure time, compared with 3D CRT. In addition, the number of MUs is usually higher with IMRT than with VMAT. This parameter depends on the number of fields or arcs, optimization parameters, and planning systems as well as on the concerned physicist and their experience and qualifications [25]. In some cases, the difference in exposure time between technologies may be minimal due to the simplicity and the small volume of the irradiated area as in the case of irradiation of only the breast, for example. With complex forms of PTV and the proximity of critical organs, the number of modulations increases greatly, as a result of which, during IMRT, the number of MUs increases greatly and, accordingly, the exposure time increases. VMAT avoids this because numerous beam modulations occur from each angle as the gantry moves along the arc, which minimizes exposure time and gives a more conformal dose distribution. When there is no CBCT option available only 3D CRT should be used.
In our study, planning was carried out in the eclipse system, and it was noted that planning with more complex technologies such as IMRT and VMAT might take longer than with 3D CRT, which should also be taken into account in practical work. Based on all of the above, we concluded that there is no universal irradiation method that can be used in every case. Radiation oncologist should choose the best treatment for each patient, taking into account his characteristics, stage and side of irradiation. However, with a high workload on the department and in cases where it is not possible to achieve the best dose distribution with the 3D CRT method, one should opt for VMAT over IMRT. To the best of our knowledge, the clinical and dosimetry analysis of 3D CRT, IMRT and VMAT for different stages and sides of breast cancer has not been studied.
Acknowledgements
Not applicable.
Funding
None.
Data Availability
Аvailable upon request.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by *****. The first draft of the manuscript was written by **** and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics Approval
This is an observational study. The Local Research Ethics Committee has confirmed that no ethical approval is required.
Consent to Participate
Informed consent was obtained from the patients.
Consent to Publish
Written consent for publication of this article was obtained from the patients, including all images.
Competing Interests
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 15, May 2023Accepted: Wed 31, May 2023
Published: Mon 19, Jun 2023
Copyright
© 2023 Kristina Tumanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RCO.2023.01.01
Author Info
Salim Nidal Kristina Tumanova Alexey Popodko Alexander Stolbovoy
Corresponding Author
Kristina TumanovaDepartment of Radiation Oncology, European Medical Center, Moscow, Russia
Figures & Tables
Table 1: Average dose distribution (Dmean)
and standard deviation (SD) in the OARs of 3D CRT, IMRT and VMAT depending on
the treatment volume.
|
|
|
Organ
at risk |
|||||||||||
|
|
|
Contralateral
Breast |
RCA |
LADA |
Myocardium |
Contralateral
Lung |
Ipsilateral
Lung |
||||||
|
Treatment
Volume |
Technique |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
Mean,
Gy |
SD |
|
Right
Breast with lymphnodes |
3D
CRT |
0,94 |
0,59 |
4,20 |
3,56 |
0,55 |
0,24 |
1,23 |
0,36 |
0,36 |
0,17 |
17,71 |
2,98 |
|
IMRT |
2,82 |
1,23 |
9,99 |
5,74 |
4,44 |
3,42 |
5,23 |
2,25 |
2,54 |
1,10 |
14,15 |
2,39 |
|
|
VMAT |
4,06 |
0,99 |
10,26 |
4,56 |
6,22 |
2,80 |
5,22 |
2,17 |
3,56 |
0,88 |
10,83 |
1,99 |
|
|
Left
Breast with lymphnodes |
3D
CRT |
0,80 |
0,77 |
3,20 |
2,88 |
19,72 |
13,73 |
7,00 |
3,95 |
0,41 |
0,34 |
16,29 |
4,63 |
|
IMRT |
2,78 |
1,43 |
7,29 |
3,59 |
12,32 |
4,88 |
7,50 |
2,80 |
2,87 |
1,23 |
12,93 |
2,21 |
|
|
VMAT |
3,72 |
1,42 |
9,66 |
5,41 |
9,33 |
4,61 |
6,10 |
2,33 |
3,53 |
1,00 |
9,83 |
1,62 |
|
|
Right
Breast |
3D
CRT |
0,26 |
0,26 |
1,47 |
0,39 |
0,18 |
0,14 |
0,54 |
0,15 |
0,09 |
0,08 |
11,24 |
4,67 |
|
IMRT |
1,36 |
0,79 |
3,88 |
2,00 |
1,48 |
1,03 |
2,41 |
1,32 |
1,23 |
0,73 |
9,83 |
2,81 |
|
|
VMAT |
2,85 |
0,99 |
8,19 |
3,11 |
4,72 |
1,51 |
5,25 |
1,57 |
2,71 |
0,88 |
9,72 |
1,94 |
|
|
Left
Breast |
3D
CRT |
0,92 |
0,77 |
1,77 |
0,88 |
14,23 |
9,89 |
6,10 |
3,83 |
0,25 |
0,17 |
11,20 |
3,53 |
|
IMRT |
2,38 |
1,40 |
3,92 |
1,34 |
9,32 |
5,08 |
5,50 |
2,08 |
1,77 |
0,64 |
9,70 |
1,78 |
|
|
VMAT |
3,17 |
1,04 |
5,92 |
2,78 |
6,99 |
3,68 |
5,39 |
2,31 |
2,98 |
0,85 |
8,71 |
1,33 |
|
Table 2: P-values of 3D CRT, IMRT and VMAT multiple
(Kruskal-Wallis test) and paired group (Tukey’s HSD test) comparison depending
on the treatment volume.
|
Organ
at risk |
|||||||
|
Treatment
Volume |
p-value |
Contralateral
Breast |
RCA |
LADA |
Myocardium |
Contralateral
Lung |
Ipsilateral
Lung |
|
Right
Breast with lymphnodes |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
3D
vs IMRT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
|
IMRT
vs VMAT |
<0.001 |
0,978 |
0,037 |
0,995 |
<0.001 |
<0.001 |
|
|
Left
Breast with lymphnodes |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
0,006 |
0,176 |
<0.001 |
<0.001 |
|
3D
vs IMRT |
<0.001 |
0,052 |
0,003 |
0,697 |
<0.001 |
<0.001 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
<0.001 |
0,676 |
<0.001 |
<0.001 |
|
|
IMRT
vs VMAT |
0,006 |
0,042 |
0,41 |
0,227 |
0,006 |
<0.001 |
|
|
Right
Breast |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
0,718 |
|
3D
vs IMRT |
0,001 |
0,068 |
0,022 |
0,013 |
<0.001 |
0,625 |
|
|
3D
vs VMAT |
<0.001 |
0,001 |
<0.001 |
<0.001 |
<0.001 |
0,55 |
|
|
IMRT
vs VMAT |
<0.001 |
0,262 |
<0.001 |
0,009 |
<0.001 |
0,992 |
|
|
Left
Breast |
3D vs IMRT vs VMAT |
<0.001 |
<0.001 |
0,245 |
0,897 |
<0.001 |
0,187 |
|
3D
vs IMRT |
0,004 |
0,039 |
0,254 |
0,885 |
<0.001 |
0,355 |
|
|
3D
vs VMAT |
<0.001 |
<0.001 |
0,06 |
0,842 |
<0.001 |
0,07 |
|
|
IMRT
vs VMAT |
0,414 |
0,057 |
0,723 |
0,995 |
<0.001 |
0,634 |
|

References
1. (2021) Breast
cancer. World Health Organization.
2. National
Comprehensive Cancer Network (NCCN) (2022) Clinical Practice Guidelines in
Oncology. Breast Cancer.
3. Early Breast Cancer
Trialists' Collaborative Group (2000) Favourable and unfavourable effects on
long-term survival of radiotherapy for early breast cancer: an overview of the
randomised trials. Lancet 355: 1757-1770
4. Li ZW, Zhang M,
Yang YJ, Zhou ZJ, Liu YL et al. (2020) Radiotherapy after mastectomy has
significant survival benefits for inflammatory breast cancer: a SEER
population-based retrospective study. PeerJ. 8: e8512. [Crossref]
5. Remick J, Amin NP
(2023) Postmastectomy Breast Cancer Radiation Therapy. StatPearls [Internet].
[Crossref]
6. Huang YJ, Huang TW,
Lin FH, Chung CH, Tsao CH et al. (2017) Radiation Therapy for Invasive Breast
Cancer Increases the Risk of Second Primary Lung Cancer: A Nationwide
Population-Based Cohort Analysis. J. Thorac Oncol 12: 782-790. [Crossref]
7. Jutzy JMS, Lemons
JM, Luke JJ, Chmura SJ (2018) The Evolution of Radiation Therapy in Metastatic
Breast Cancer: From Local Therapy to Systemic Agent. Int J Breast Cancer
2018: 4786819. [Crossref]
8. Esen CSB, Gultekin
M, Yildiz F (2022) Role of radiotherapy in oligometastatic breast cancer:
Review of the literature. World J Clin Oncol 13: 39-48. [Crossref]
9. Xie L, Lin C, Zhang
H, Bao X (2018) Second malignancy in young early-stage breast cancer patients
with modern radiotherapy: A long-term population-based study (A
STROBE-compliant study). Medicine 97: 1-8. [Crossref]
10. Zhang W, Becciolini
A, Biggeri A, Pacini P, Muirhead CR (2011) Second malignancies in breast cancer
patients following radiotherapy: a study in Florence, Italy. Breast Cancer
Res 13: R38. [Crossref]
11. Taylor CW, Kirby AM
(2015) Cardiac Side-effects From Breast Cancer Radiotherapy. Clin Oncol (R
Coll Radiol) 27: 621-629. [Crossref]
12. Castaneda SA,
Strasser J (2017) Updates in the Treatment of Breast Cancer with Radiotherapy. Surg
Oncol Clin N Am 26: 371-382 (2017) [Crossref]
13. Bradley JA,
Mendenhall NP (2018) Novel Radiotherapy Techniques for Breast Cancer. Annu
Rev Med 69: 277-288. [Crossref]
14. Rana S (2013)
Intensity modulated radiation therapy versus volumetric intensity modulated arc
therapy. J Med Radiat Sci 60: 81-83. [Crossref]
15. Delaney G (2005)
Recent advances in the use of radiotherapy to treat early breast cancer. Curr
Opin Obstet Gynecol 17: 27-33. [Crossref]
16. Kopchick B, Xu H,
Niu Y, Becker S, Qiu X et al. (2020) Technical Note: Dosimetric feasibility of
lattice radiotherapy for breast cancer using GammaPod. Med Phys 47:
3928-3934. [Crossref]
17. Dong J, Yang Y, Han
D, Zhao Q, Liu C et al. (2021) Hypofractionated Simultaneous Integrated Boost
Radiotherapy Versus Conventional Fractionation Radiotherapy of Early Breast
Cancer After Breast-Conserving Surgery: Clinical Observation and Analysis. Technol
Cancer Res Treat 20: 153. [Crossref]
18. Marrazzo L, Redapi
L, Zani M, Calusi S, Meattini I et al. (2022) A semi-automatic planning
technique for whole breast irradiation with tangential IMRT fields. Phys Med
98: 122-130. [Crossref]
19. Nicosia L, Figlia
V, Ricottone N, Cuccia F, Mazzola et al. (2022) Stereotactic body radiotherapy
(SBRT) and concomitant systemic therapy in oligoprogressive breast cancer
patients. Clin Exp Metastasis 39:581-588. [Crossref]
20. RTOG protocol 1005.
A phase III trial of accelerated whole breast irradiation with
hypofractionation plus concurrent boost versus standard whole breast
irradiation plus sequential boost for early-stage breast cancer.
21. Ding J, Guo Y, Li
Q, Chen J, Hu P et al. (2018) The incidence of postoperative
radiotherapy-induced acute dermatitis in breast cancer and its influencing
factors for Chinese women. Onco Targets Ther 11: 1665–70. [Crossref]
22. Abo Madyan Y, Aziz
MH, Aly MMOM, Schneider F, Sperk E et al. (2014) Second cancer risk after 3D
CRT, IMRT and VMAT for breast cancer. Radiother Oncol 110: 471-476. [Crossref]
23. Darby SC, Ewertz M,
McGale P, Bennet AM, Blom Goldman U et al. (2013) Risk of ischemic heart
disease in women after radiotherapy for breast cancer. N Engl J Med 368:
987–98. [Crossref]
24. Liu H, Chen X, He
Z, Li J (2016) Evaluation of 3D CRT, IMRT and VMAT radiotherapy plans for left
breast cancer based on clinical dosimetric study. Comput Med Imaging Graph
54: 1-5. [Crossref]
25. Ma C, Zhang W, Lu
J, Wu L, Wu F et al. (2015) Dosimetric Comparison and Evaluation of Three
Radiotherapy Techniques for Use after Modified Radical Mastectomy for Locally
Advanced Left-sided Breast Cancer. Sci Rep 5: 12274. [Crossref]
26. Das Majumdar SK,
Amritt A, Dhar SS, Barik S, Beura SS et al. (2022) A Dosimetric Study Comparing
3D-CRT vs. IMRT vs. VMAT in Left-Sided Breast Cancer Patients After Mastectomy
at a Tertiary Care Centre in Eastern India. Cureus 14: e23568. [Crossref]
27. Ahmad A, Das S,
Kharade V, Gupta M, Pandey VP et al. (2022) Dosimetric Study Comparing 3D
Conformal Radiotherapy (3D-CRT), Intensity Modulated Radiotherapy (IMRT) and
Volumetric Modulated Arc Therapy (VMAT) in Hypofractionated One-Week
Radiotherapy Regimen in Breast Cancer. Cureus. 14: e31860. [Crossref]
28. Mayo CS, Urie MM,
Fitzgerald TJ (2005) Hybrid IMRT plans-concurrently treating conventional and
IMRT beams for improved breast irradiation and reduced planning time. Int J
Radiat Oncol Biol Phys 61: 922-932. [Crossref]
29. Lin JF, Yeh DC, Yeh
HL, Chang CF, Lin JC (2015) Dosimetric comparison of hybrid
volumetric-modulated arc therapy, volumetric-modulated arc therapy, and
intensity-modulated radiation therapy for left-sided early breast cancer. Med
Dosim 40: 262-267. [Crossref]
30. Chen YG, Li AC, Li WY, Huang MY, Li XB et al. (2017) The Feasibility Study of a Hybrid Coplanar Arc Technique Versus Hybrid Intensity-modulated Radiotherapy in Treatment of Early-stage Left-sided Breast Cancer with Simultaneous-integrated Boost. J Med Phys 42: 1-8. [Crossref]
31. Smith S, Estoesta R, Kader J. Martin D, Claridge Mackonis ER et al. (2016) Hybrid intensity-modulated radiation therapy (IMRT) simultaneous integrated boost (SIB) technique versus three-dimensional (3D) conformal radiotherapy with SIB for breast radiotherapy: A planning comparison. Journal of Radiotherapy in Practice 15: 131-142.
