Multimodal and Mini-Invasive Treatment of a Multifocal Metastatic Mid-Gut Neuroendocrine Tumor: A Surgical Case Report
Multimodal and Mini-Invasive Treatment of a Multifocal Metastatic Mid-Gut Neuroendocrine Tumor: A Surgical Case Report
A B S T R A C T
Neuroendocrine tumors represent the most common small bowel malignancy. At the time of presentation, they often debut as metastatic disease. Consensus guidelines recommend a multimodal and multidisciplinary approach that includes punctual investigations, aggressive surgical resection of the primary tumor and the assessment of possible synchronous metastasis through surgical procedures or ablative techniques. This report details the case of a 77-year-old male with a multifocal mid-gut neuroendocrine tumor with nodal dissemination and synchronous hepatic metastasis. He underwent a laparoscopic procedure that included an ileal resection with intracorporeal side-to-side ileo-ileal anastomosis, a large mesenteric nodal dissection extended up to superior mesenteric vein, a laparoscopic dissection of hepatic metastasis and an ultra-sound-guided thermal ablation of two hepatic metastasis. This case highlights the efficacy of laparoscopy, providing a mini-invasive radical treatment and the importance of an aggressive multimodal approach in facing a metastatic multifocal mid-gut neuroendocrine tumor.
Keywords
Neuroendocrine tumors, nodal dissection, laparoscopic ileal dissection
Introduction
Neuroendocrine tumors (NETs) are a rare heterogeneous type of tumor that arises from specialized cells of the neuroendocrine system, dispersed throughout the body. Based on their embryogenic origin, they are classified into foregut NETs (bronchial, thymic, gastric, duodenal, pancreatic), midgut NETs (ileal, jejunal, caecal) and hindgut NETs (distal colonic, rectal) [1-3]. In recent years there has been an increased incidence of NETs and midgut NETs represent 26% of diagnosed NETs resulting in now the most common small bowel malignancy [3-6]. Midgut NETs can grow slowly, and they often remain asymptomatic for a long time. For this reason, early diagnosis is a challenge and it is quite common to diagnose a midgut NET in advanced stage [7, 8]. In addition, the debut of midgut NETs is characterized in about 45% of cases by multifocality of the primary tumor [6, 9].
Consensus recommendations suggest a multimodal and multidisciplinary approach, with a radical treatment by surgery of primary lesions and metastasis and ablative techniques of metastasis [7, 10, 11]. In the past, some authors doubted the use of laparoscopy for midgut NETs because of the need for the manual palpation for correct identification of the smaller primary lesions, the extensive nodal dissection, and the assessment of possible synchronous lesions. However, it is now widely known how in selected patient laparoscopic surgery allows to get oncological efficacy with a mini-invasive approach for these tumors too [12-14]. This case highlights the importance of a multimodal approach with a multifocal metastatic NET and the efficacy of laparoscopy in achieving a radical treatment maintaining minimally invasive access. This case is reported in accordance with the SCARE checklist [15].
Case Presentation
A 77-year-old male, with body mass index 29.41 kg/m² and a past medical history of hypertension, hypercholesterolemia, ex-smoker, underwent clinical investigations for a traumatic thoracic-abdominal accident and a contrast tomography showed nonspecific lymph-nodes spread along the mesenteric root and a sub-centimeter hepatic lesion in the V segment of liver (according to Couinaud classification), suspected to be hepatic hemangioma but worthy of further investigations. An abdominal magnetic resonance imaging (MRI) revealed enlarged mesenteric lymph-nodes and a 12 mm in diameter lesion in the VI-VII segment of the liver.
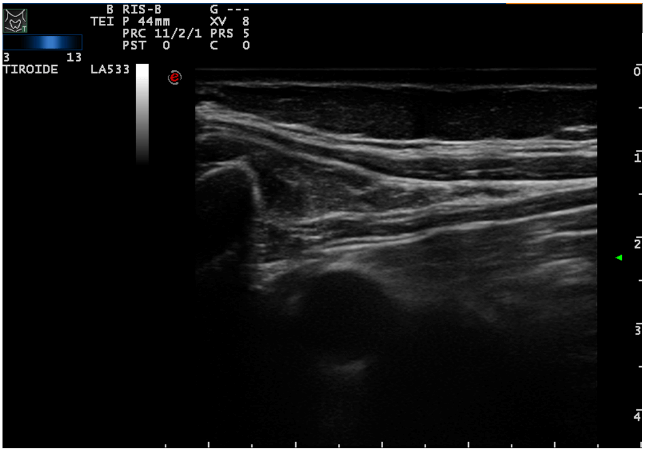
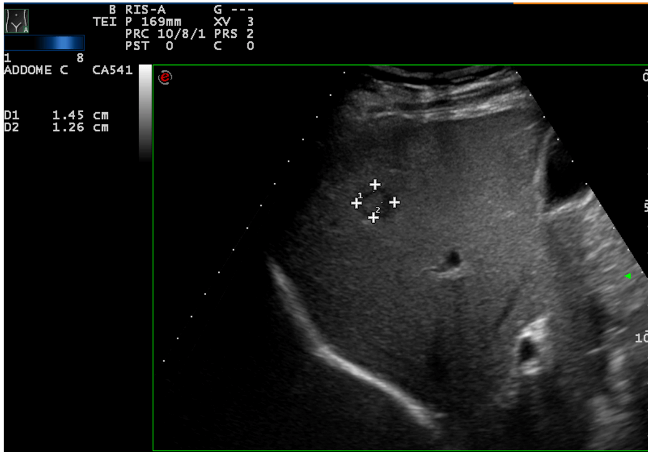
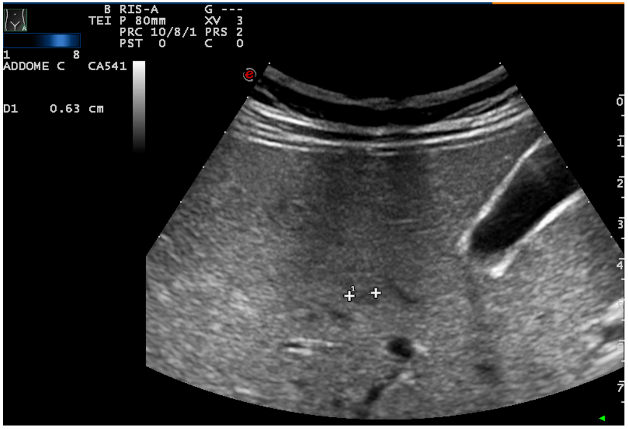
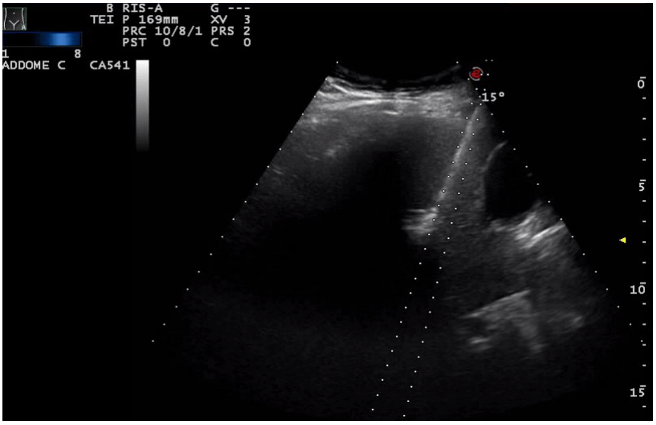
The patient underwent a contrast-enhanced ultrasound (CEUS) and an ultrasound-guided biopsy. The histological examination revealed a metastatic localization of gastro-enteric NET, G1 (ki67<2%). A positron emission tomography (PET) with 68-DOTA scan was undertaken and it showed two focal areas of increased uptake in mesenteric lymph-nodes, an avid lesion in the right lobe of the liver (VI-VII segment) and also 3 areas of uptake spread along the loops of the small intestine. Urinary 5-HIAA and Chromogranin A levels were normal. During re-staging, CEUS and MRI confirmed a multifocal ileal neuroendocrine tumor with lymph-nodal dissemination in the mesentery (Figure 1) and a metastasis in the VI-VII segment of the liver (Figure 2). These examinations also showed two new lesions in the V segment of the liver (Figure 3), suspected to be metastasis.
Figure 1: Nodal dissemination in mesenteric leaf.
Figure 2: Hepatic lesion in the VI-VII segment of the liver.
Figure 3: Hepatic lesion in the V segment of the liver.
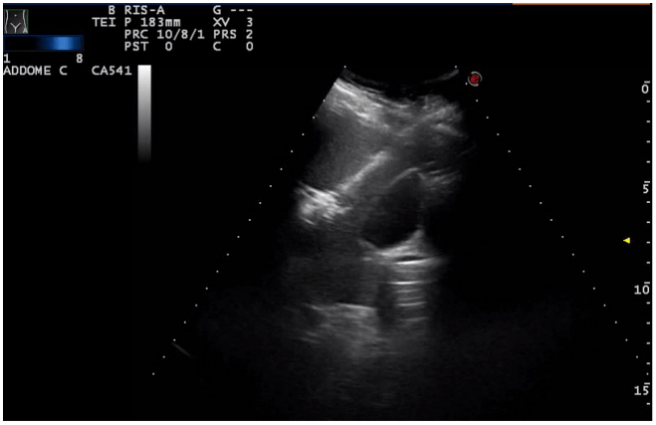
In our department, the patient underwent a multidisciplinary approach involving laparoscopic surgery and percutaneous ultrasound-guided thermal-ablation of intrahepatic metastasis. In the operating room, the patient was positioned supine. The assessment of the intrahepatic metastasis was addressed by two oncologists specialized in ultra-sound-guided procedures and the surgery was undertaken by two experienced surgeons and a resident in General Surgery. The technical approach started with the examination and identification of the intraparenchymal hepatic metastasis using first ultra-sound (US) and then CEUS. We proceeded with the US-guided percutaneous microwave thermal-ablation of the hepatic lesion in the VI segment with an intercostal approach, using a 14 Gauge “Antenna” needle with microwave energy supply (2.45 MHz) at a power of 50 Watt for 8 minutes (Figure 4). The lesion in the V segment underwent thermal ablation using the same needle for 6 minutes at a power of 40 Watt (Figure 5).
Figure 4: Percutaneous US-guided thermal ablation of the lesion of the VII segment.
Figure 5: Percutaneous US-guided thermal ablation of the lesion of the V segment.
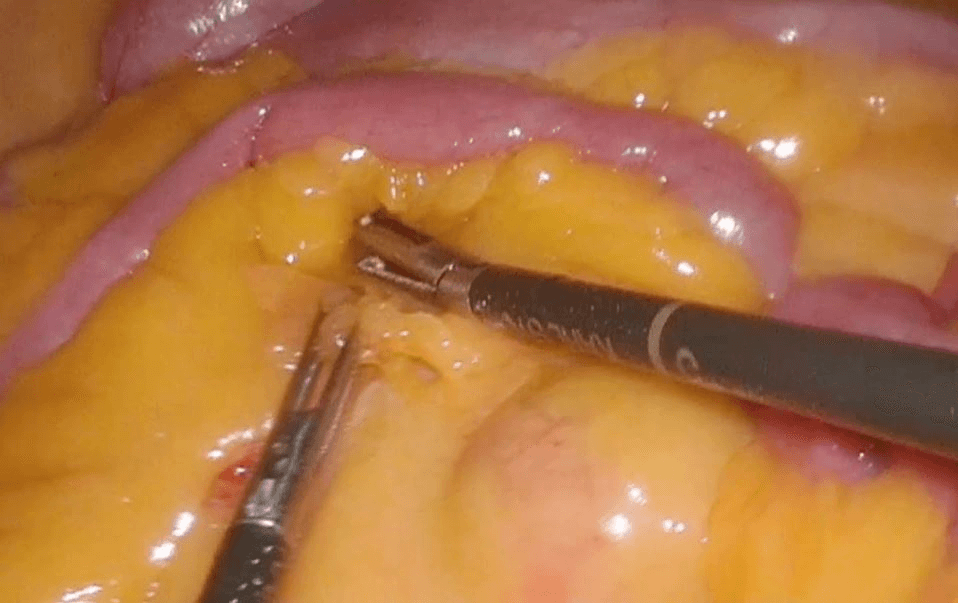
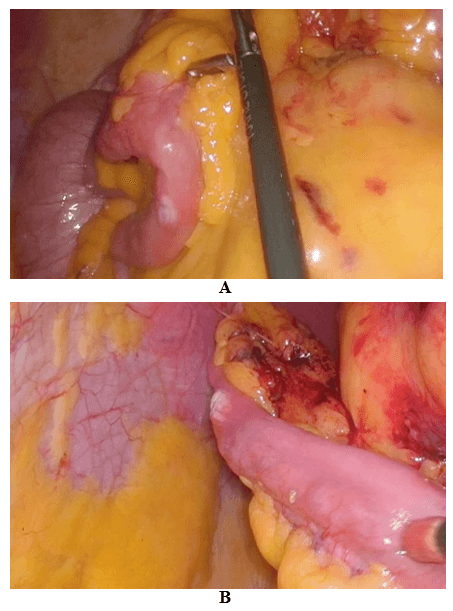
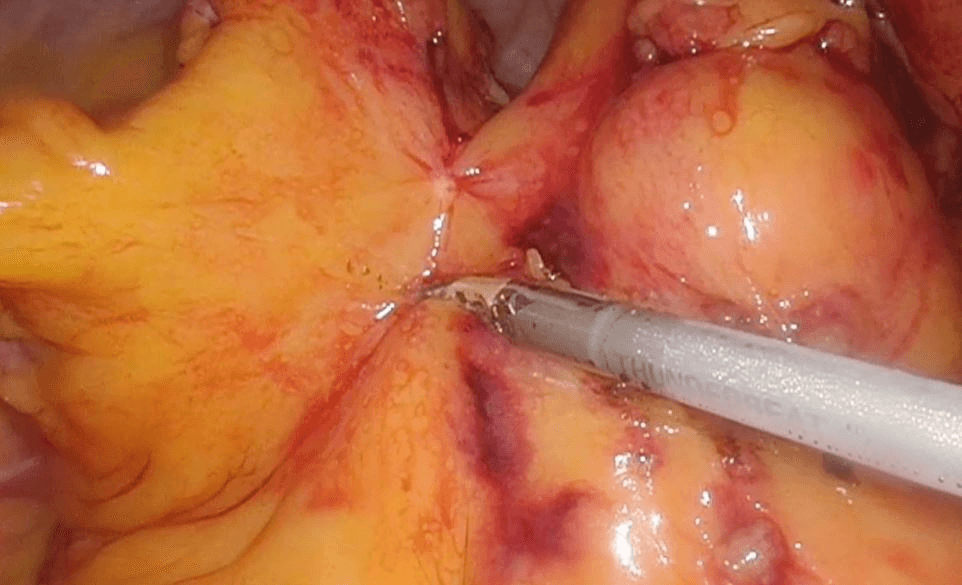
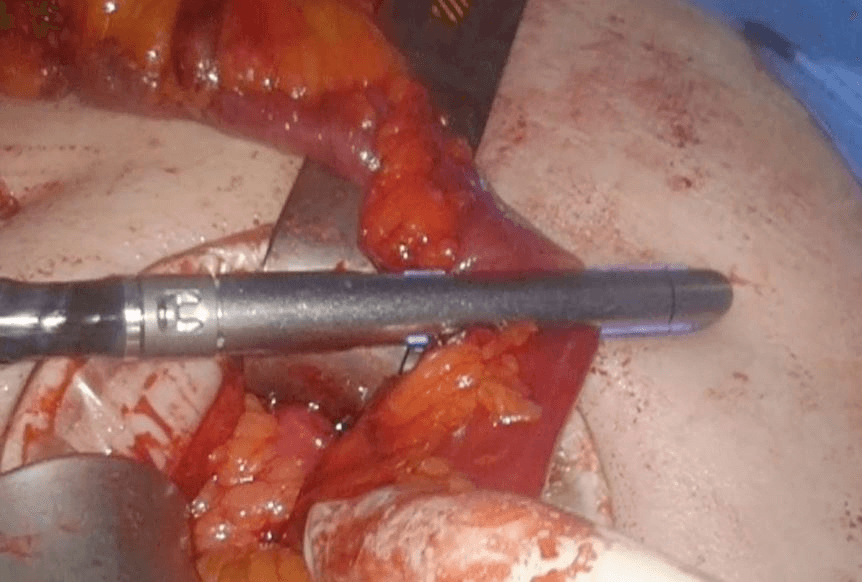
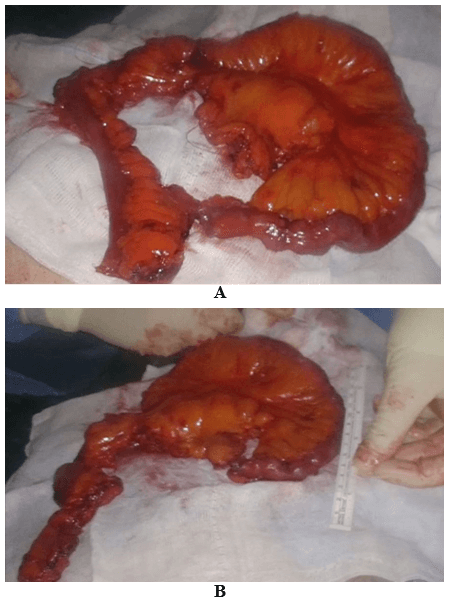
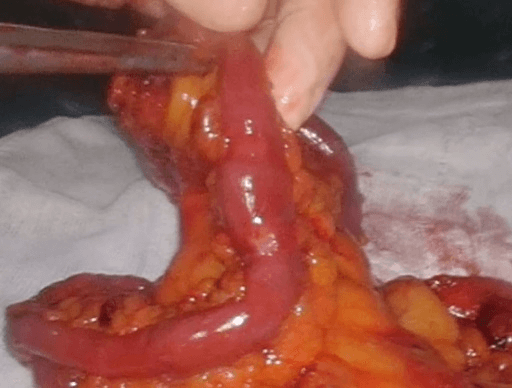
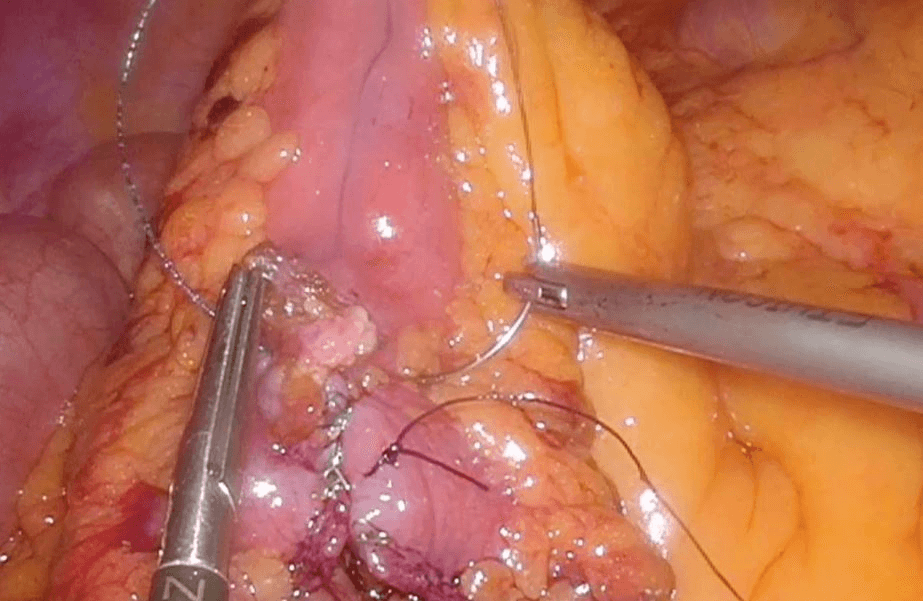
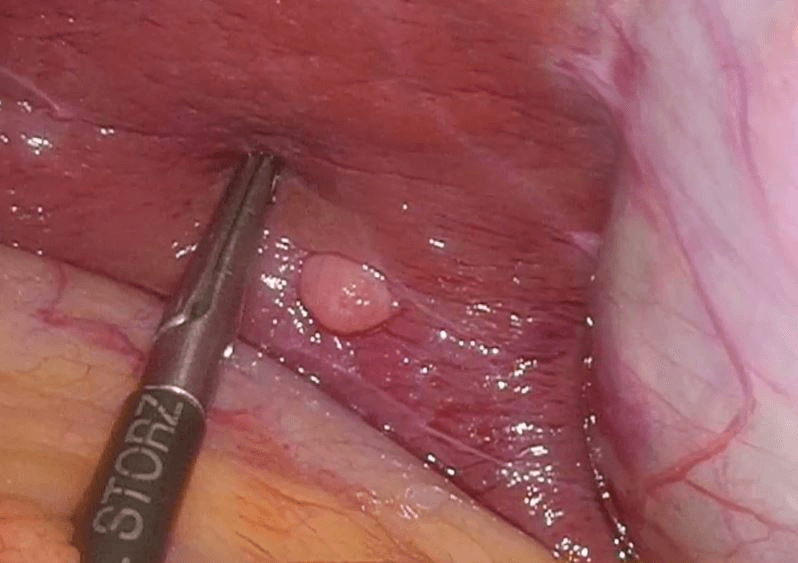
Then laparoscopy was performed using four trocars: a periumbilical incision for the Hasson trocar, a 12 mm trocar in left hypochondrium for first operator’s right hand, two 5 mm port in the epigastrium and in left iliac fossa for the first-operator’s left hand and for retracting instruments. On initial exploration, of the abdominal cavity we immediately visualized a bulky nodal lesion, tough to the touch with laparoscopic instruments, situated in the mesentery root nearby superior mesenteric vessels (Figure 6). During abdominal exploration we also could identify several ileal lesions of the primary tumor (Figures 7A & 7B). A large mesenteric nodal dissection extended up to a superior mesenteric vein was undertaken using an ultrasonic dissector (Figure 8). Through an enlargement of the umbilical port-site, a mini-laparotomy was created to remove the surgical specimen of 60 centimeters, allowing an accurate extracorporeal manual palpation of intestinal loops furthermore to ensure a complete resection of neuroendocrine lesions (Figures 9, 10A, 10B & 11). A laparoscopic side-to-side ileo-ileal intracorporeal anastomosis was performed using a 60 mm blue load Echelon Stapler (Figure 12) and 3-0 V-Loc suture for the mesenteric structures (Figure 13).
Figure 6: Nodal lesion in mesenteric root.
Figure 7: A) & B) Ileal lesion.
Figure 8: Large mesenteric dissection with US-dissector.
Figure 9: Exteriorization and resection of the surgical specimen after an accurate palpation.
Figure 10: A) & B) Surgical specimen.
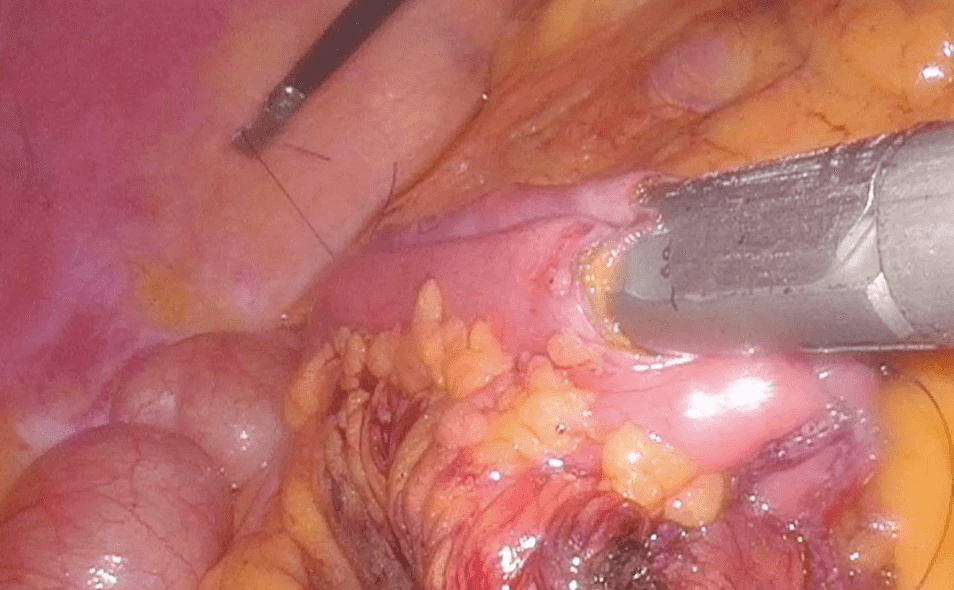
During this laparoscopic procedure, an ulterior hepatic lesion in the V segment was identified and it was removed surgically using ultrasonic dissector (Figure 14). Two abdominal drains were inserted beneath the liver and along the right parietocolic wall and wounds were sutured. The postoperative course was uneventful, the patient resumed eating during the first postoperative day, the drains were removed in the second postoperative day and he was discharged on the sixth postoperative day. Three months later, a CT showed no disease recurrence neither in the liver nor in the lymph-glandular level. This examination also demonstrated an asymptomatic non-occlusive thrombosis of the mesenteric vein, treated with low-molecular-weight heparin.
Figure 11: Multiple ileal lesion on the surgical specimen.
Figure 12: Laparoscopic side-to-side ileo-ileal intracorporeal anastomosis with 60 mm blue load Echelon Stapler.
Figure 13: 3-0 V-Loc suture for the mesenteric structures.
Figure 14: Ulterior hepatic lesion in the V segment surgically removed using ultrasonic dissector.
Discussion
The authors illustrate a clinical example of midgut multifocal neuroendocrine tumor with loco-regional nodal metastasis and hepatic metastasis, radically treated with a multimodal approach. The patient was asymptomatic, and the hepatic metastasis were accidentally diagnosed. As a result of a biopsy of the bigger hepatic lesion, further investigations (CT, PET and intestinal MRI) were undertaken. These examinations demonstrated the origin from a multifocal neuroendocrine tumor located along the middle ileum, with loco-regional nodal dissemination.
As literature reports, NETs grow slowly and they can remain asymptomatic for a long time; for this reason, it is frequent to observe a metastatic disease at the time of diagnosis (from 35 to 60% of cases) and it is quite common to diagnose metastasis of unknown primary NET. However, despite this advanced presentation at the time of diagnosis, a good survival rate (median survival of 56 months) can be observed in patients with metastatic disease and it can be improved further by treatment [7, 8, 16]. For what concern patients with liver metastasis of mid-gut NET (that occur in 27% of cases at presentation), multivariate analyses identify the age at diagnosis, low ki67 index (<2%) and the resection of the primary tumor as independent predictors of survival. Moreover, the resection of the liver metastasis is associated with improved survival [10].
In 25% of cases, midgut NETs arise as multifocal lesions and several studies show the absence of remarkable differences in clinical course, survival, or recurrence outcome between multifocal and unifocal lesions [6, 9]. North American Neuroendocrine tumors society recommends an aggressive multimodal and multidisciplinary treatment, requiring complete surgical resection of multifocal lesions of the primary tumor, extensive lymph-node dissection and the assessment of possible synchronous metastatic lesions [7, 10, 11, 14]. For what concern the treatment of liver metastasis, surgical resection remains the gold standard, achieving a better survival rate and quality of life, but the use of intraoperative ultrasonography (US) and local ablative techniques may help to ensure a complete removal of liver metastasis [17-21].
In the clinical example illustrated, metastasis was few and small; the superficial hepatic metastasis was resected using US dissector in laparoscopy, while the ones within the hepatic parenchyma were treated using radiofrequency ablation. This surgical and ablative treatment was oncologically radical and respectful of the safe parenchyma to obtain oncological efficacy maintaining a mini-invasive approach. Also, in respect of mini-invasiveness, 3D laparoscopic view was used to achieve accurate wide ileal resection with nodal dissection and central vascular ligation. Some authors would advise laparoscopy again in this case, preferring open surgery to achieve a punctual identification of primary lesions by manual palpation. Actually a safe surgery can be reached with high sensitivity of pre-operative investigations, such as PET and abdominal RMI, and through the help of periumbilical mini-laparotomy, that allows exteriorization and manual palpation of intestinal loops, that authors performed to verify correct localization of multiple lesions and to remove the surgical specimen. Lymphadenectomy with central vascular ligation, which is mandatory, can be performed very carefully using 3D view.
In author’s opinion, the non-occlusive thrombosis of the superior mesenteric vein (SMV) was a complication due to the manipulation of vessels during central vascular ligation, elevated intra-abdominal pressure generated by the pneumoperitoneum and the presence of intra-abdominal malignancy, which are predisposing factors of portomesenteric venous thrombosis [22-24]. The construction of side-to-side ileo-ileal anastomosis was intracorporeal because intracorporeal anastomosis is regarded as superior to the extracorporeal one in terms of recovery of peristalsis, analgesia requirements and length of hospital stay [25-28]. In conclusion, the treatment of multifocal mid-gut NET with metachronous nodal and hepatic metastasis should be multimodal with a mini-invasive but radical target. Laparoscopy is a safe and effective technique, which can achieve the same guarantees of open surgery.
Data Availability
The data supporting conclusions can be found in literature following the references.
Conflicts of Interest
None.
Funding
No funding was received in support of the study; it was performed as part of the employment of the authors at the clinic “Istituti Clinici di Pavia e Vigevano”, in Pavia, Italy.
Acknowledgments
None.
Article Info
Article Type
Case ReportPublication history
Received: Tue 04, Aug 2020Accepted: Tue 18, Aug 2020
Published: Mon 31, Aug 2020
Copyright
© 2023 Annamaria Ruggieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.08.21
Author Info
Annamaria Ruggieri Fabrizio Gambarini Carlo D’Angelo Benedetta Montagna Guido Poggi Mario Martinotti
Corresponding Author
Annamaria RuggieriDepartment of General Surgery of Istituto di Cura Città di Pavia, Pavia, Italy
Figures & Tables
References
- E D Williams, M Sandler (1963) The classification of carcinoid tumours. Lancet 1: 238-239. [Crossref]
- Vincent T F Cheung, Mohid S Khan (2015) A guide to midgut neuroendocrine tumours (NETs) and carcinoid syndrome. Frontline Gastroenterol 6: 264-269. [Crossref]
- Amir Mehrvarz Sarshekeh, Daniel M Halperin, Arvind Dasari (2016) Update on management of midgut neuroendocrine tumors. Int J Endocr Oncol 3: 175-189. [Crossref]
- Karl Y Bilimoria, David J Bentrem, Jeffrey D Wayne, Clifford Y Ko, Charles L Bennett et al. (2009) Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 249: 63-71. [Crossref]
- Lorna R Woodbridge, Bernadine M Murtagh, Dominic F Q C Yu, Katie L Planche (2014) Gastrointestinal Imaging. Midgut Neuroendocrine Tumors: Imaging Assessment for Surgical Resection. Radiographics 34: 413-426. [Crossref]
- Alexandra Gangi, Emily Siegel, Galinos Barmparas, Simon Lo, Laith H Jamil et al. (2018) Multifocality in small bowel neuroendocrine tumors. J Gastrointest Surg 22: 303-309. [Crossref]
- James R Howe, Kenneth Cardona, Douglas L Fraker, Electron Kebebew, Brian R Untch et al. (2017) The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American neuroendocrine tumor society. Pancreas 46: 715-731. [Crossref]
- Linee guida AIOM neoplasie neuroendocrine (October 2019).
- Allen B Choi, Jessica E Maxwell, Kendall J Keck, Andrew J Bellizzi, Joseph S Dillon et al. (2017) Is Multifocality an Indicator of Aggressive Behavior in Small Bowel Neuroendocrine Tumors? Pancreas 46: 1115-1120. [Crossref]
- A Ahmed, G Turner, B King, L Jones, D Culliford et al. (2009) Midgut Neuroendocrine Tumours With Liver Metastases: Results of the UKINETS Study. Endocr Relat Cancer 16: 885-894. [Crossref]
- Jonathan R Strosberg, Thorvardur R Halfdanarson, Andrew M Bellizzi, Jennifer A Chan, Joseph S Dillon et al. (2017) The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 46: 707-714. [Crossref]
- Kristen P Massimino, Esther Han, SuEllen J Pommier, Rodney F Pommier (2012) Laparoscopic surgical exploration is an effective strategy for locating occult primary neuroendocrine tumors. Am J Surg 203: 628-631. [Crossref]
- Petachia Reissman, Shmail Shmailov, Simona Grozinsky Glasberg, David J Gross (2013) Laparoscopic resection of primary midgut carcinoid tumors. Surg Endosc 27: 3678-3682. [Crossref]
- Marleny N Figueiredo, Léon Maggiori, Sébastien Gaujoux, Anne Couvelard, Nathalie Guedj et al. (2014) Surgery for small-bowel neuroendocrine tumors: is there any benefit of the laparoscopic approach? Surg Endosc 28: 1720-1726. [Crossref]
- Riaz A Agha, Alexander J Fowler, Alexandra Saeta, Ishani Barai, Shivanchan Rajmohan et al. (2016) The SCARE Statement: Consensus-based surgical case report guidelines. Int J Surg 34: 180-186. [Crossref]
- Mauro Cives, Jonathan R Strosberg (2018) Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin 68: 471-487. [Crossref]
- Timothy M Pawlik, Francesco Izzo, Deborah S Cohen, Jeffery S Morris, Steven A Curley (2003) Combined Resection and Radiofrequency Ablation for Advanced Hepatic Malignancies: Results in 172 Patients. Ann Surg Oncol 10: 1059-1069. [Crossref]
- Thomas Steinmüller, Reza Kianmanesh, Massimo Falconi, Aldo Scarpa, Babs Taal et al. (2008) Consensus Guidelines for the Management of Patients with Liver Metastases from Digestive (Neuro)endocrine Tumors: Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 87: 47-62. [Crossref]
- Loukia S Poulou, Panayiotis D Ziakas, Vassilia Xila, George Vakrinos, Katerina Malagari et al. (2009) Percutaneous Radiofrequency Ablation for Unresectable Colorectal Liver Metastases: Time for Shadows to Disperse. Rev Recent Clin Trials 4: 140-146. [Crossref]
- Marianne Pavel, Eric Baudin, Anne Couvelard, Eric Krenning, Kjell Öberg et al. (2012) ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 95: 157-176. [Crossref]
- Philipp Kron, Michael Linecker, Robert P Jones, Giles J Toogood, Pierre-Alain Clavien et al. (2019) Ablation or Resection for Colorectal Liver Metastases? A Systematic Review of the Literature. Front Oncol 9: 1052. [Crossref]
- Aaron W James, Charlotte Rabl, Antonio C Westphalen, Patrick F Fogarty, Andrew M Posselt et al. (2009) Portomesenteric Venous Thrombosis After Laparoscopic Surgery A Systematic Literature Review. Arch Surg 144: 520-526. [Crossref]
- Iswanto Sucandy, Jon D Gabrielsen, Anthony T Petrick (2010) Postoperative mesenteric venous thrombosis: Potential complication related to minimal access surgery in a patient with undiagnosed hypercoagulability. N Am J Med Sci 2: 329-332. [Crossref]
- Ramesh Kumar, Namrata Choudary (2017) Mesenteric Vein Thrombosis after Laparoscopic Right Colectomy - A Case Report and Literature Review. Cancer Surg 3: 115.
- Maude Trépanier, Anthony Valin Thorburn, Araz Kouyoumdjian, Teodora Dumitra, Mohsen Alhashemi et al. (2019) Intracorporeal Versus Extracorporeal Anastomosis for Right Colectomy Does Not Affect Gastrointestinal Recovery Within an Enhanced Recovery After Surgery Program. Surg Endosc. [Crossref]
- Intra-corporeal vs Extra-corporeal Anastomosis in Laparoscopically Assisted Right Hemicolectomy 2019.
- History of Changes for Study: Intra-corporeal vs Extra-corporeal Anastomosis in Laparoscopically Assisted Right Hemicolectomy. January 2019 on ClinicalTrials.gov.
- Matthew T Brady (2019) The advantage of intracorporeal techniques. ALES 4.
