Mutations in P63 and IRF-6 Present with Overlapping Craniofacial Defects: A study from Lebanon
A B S T R A C T
Interferon Regulatory Factor 6 (IRF-6) and p63 are two vital transcription factors implicated in normal craniofacial development. In this report, we present a family with Van Der Woude Syndrome (VWS) with a mutation in exon 9 of IRF-6 gene and a phenotypically overlapping case of Rapp-Hodgkin Syndrome (RHS) resulting from a mutation in the p63 gene. Members from both families presented with congenital lip pits and cleft lip/palate. The RHS case had additional ectodermal features that underscore the upstream nature of p63 in the complex p63-IRF-6 interactive pathway.
Keywords
Lip pits, p63-related disorders, ectodermal dysplasia, IRF-6
Introduction
The intricate nature of embryogenesis requires tightly regulated interactions between different signaling pathways. P63 is a key protein and its loss or aberrant functioning is often the major culprit in ectodermal dysplasias, particularly those presenting with cleft lip and palate (CLP). P63-related disorders are divided into six major groups, each is categorized by a constellation of phenotypic features and deleterious heterozygous mutations in p63. These six groups are at times hard to clinically differentiate as many features are overlapping, in addition there is a wide range of intra-syndromic, and intra-familial variability [1].
CLP are phenotypically evident developmental defects and thus are easy to identify. CLP are cardinal features of Ectrodactyly-Ectodermal- Dysplasia-Cleft-lip/palate (EEC) Syndrome, Ankyloblepharon- Ectodermal dysplasia-clefting (AEC) syndrome, and Rapp-Hodgkin Syndrome (a milder form of AEC), all of which are P63-related disorders. This highlights the importance of P63 in craniofacial development [1]. A second important transcription factor in craniofacial development is Interferon Regulatory Factor 6 (IRF-6), which when anomalous also results in syndromes characterized by lip/palate clefting.
There are two IRF-6- related syndromes; Van der Woude (VWS) and popliteal pterygium syndromes (PPS). These are autosomal dominant disorders categorized by the presence of lower lip pits [2]. Congenital lip pits are thought to occur from early notching of the lips with fixation of the tissue at the base of the notch or from incomplete union of embryonic lateral lip sulci. The concurrence of lip pits with cleft lip/palate points out to an overlapping embryological origin [2].
Here we present two Arab families with overlapping craniofacial features. Genetic testing identified a mutation in the IRF-6 gene in family 1 and a mutation in P63 in the second family. Family 1 was eventually diagnosed with VWS and the affected member in family 2 was diagnosed with RHS. The clinical and molecular data underscores the cross-talk/interaction between p63 and IRF-6 in craniofacial development.
I Family 1
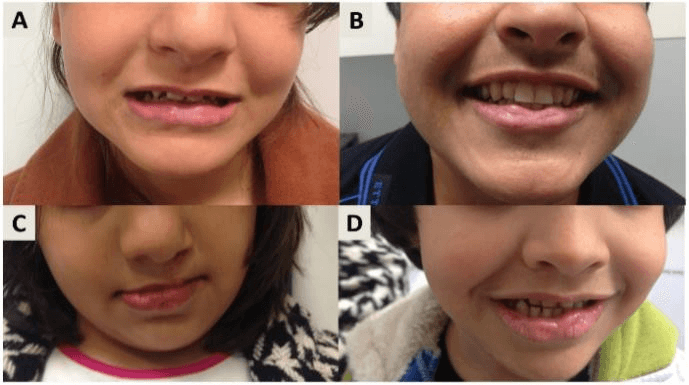
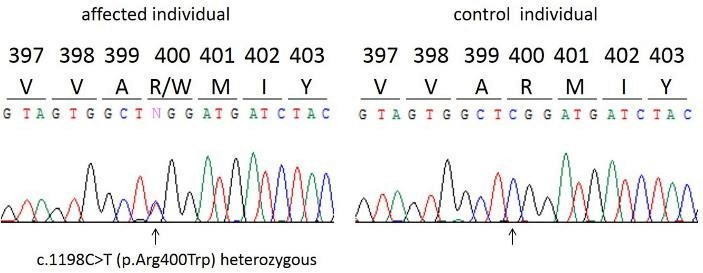
A 14- year old Iraqi girl was referred to us for bilateral lower lip transverse pits with conical elevations, associated with a corrected cleft lip/palate (Figure 1A). On further questioning she was found to have three affected siblings; a 12-year old brother with isolated lower lip pits (Figure 1B), a 10-year old sister with lower lip pits, corrected cleft lip and palate (Figure 1C), and a 7-year old sister with lower lip pits (Figure 1D). Parents were unaffected. Genetic analysis identified a recurrent heterozygous mutation in exon 9 of the IRF-6 gene (c.1198C>T), confirming the diagnosis of VWS (Figure 2). Interestingly and similarly to the affected children, the unaffected mother was also found to be a heterozygous for the mutation.
Figure 1: A) Bilateral lower lip transverse pits with conical elevations. B) Lower lip pits. C) Lower lip pits corrected cleft lip and palate (Figure 1C), D) Lower lip pits.
Figure 2: Identification of a recurrent exon 9 heterozygous mutation of the IRF-6 gene in a family with Van der Woude Syndrome.
II Family 2
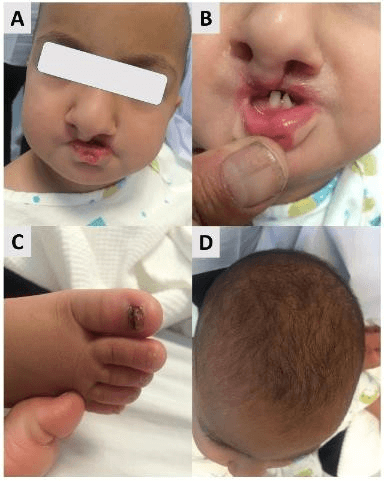
A 2-year old baby girl was admitted for the correction of a cleft palate, upon dermatology consultation for nail changes she was also found to have curly, short hair, conical teeth, lower lip pits on the paramedian vermillion border and twenty nail dystrophy (Figure 3: A-D). Genetic analysis identified a recurrent heterozygous mutation in P63, 1529C>T transition in exon 12 leading to Ile510-to-Thr (I510T) substitution. These findings confirmed the diagnosis of RHS.
Figure 3: A) Cleft palate and lip pits. B) Conical teeth. C) Dystrophic nails. D) short curly hair.
Discussion
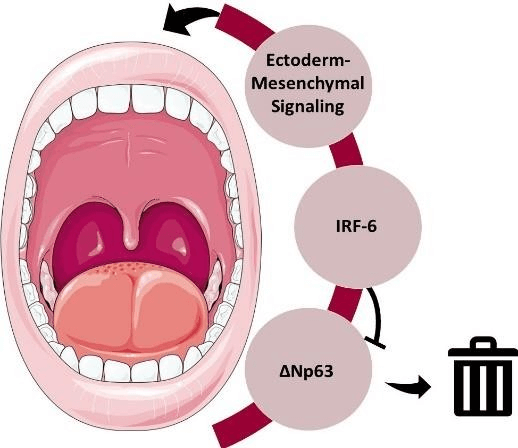
Understanding of the molecular pathways in syndromic genetic diseases has been of utmost importance in delineating embryological pathways and in unraveling signaling pathways implicated in craniofacial development. P63 is a major determinant of skin development as well as being a key regulator in ectodermal structures development. For instance, different isoforms of P63 are implicated in proliferation or differentiation of several ectodermal structures. In brief, one of those isoforms, ΔNp63, was found to cooperate with IRF-6 in the normal process of cranial development. IRF-6 was found to be a direct target of p63 and its expression requires normal function of ΔNp63. In addition, IRF-6 was also found to be responsible for the proteasomal degradation of ΔNp63, forcing the cells to differentiate and facilitate normal embryogenesis [3]. This “Regulatory loop” seems to be important in the appropriate lip and palate development (Figure 4). However, p63, being an upstream regulator of IRF-6 appears to be responsible for a wider spectrum of clinical presentations that includes other ectodermal features in addition to craniofacial defects [4]. IRF-6 dominant mutations, such as in VWS, can present as isolated pits without clefts (44%), pits with clefts (26%), only clefts (10%), or neither (20%) [5].
Figure 4: The P63- IRF-6 regulatory loop. The expression of IRF-6 requires normal function of P63 to result in the appropriate ectoderm- mesenchymal signaling (arrows) for normal craniofacial development. IRF-6 is responsible for the proteasomal degradation of ΔNp63 (blunt arrow). This balance is important to regulate cell cycling and differentiation.
In the family presented here with VDS, two of the affected members had only lower lip pits while the other two had in addition cleft lip and palate that were repaired during the first months of life. Interestingly, the mother carrying the same mutation did not have any clinical features. Therefore, we observe that there is incomplete penetrance of the disease as well as variable expressivity even among members of the same family. It is possible that multiple other genetic determinants affect the disease manifestation. Variable expressivity has been also observed in P63 mutations among individuals with ectodermal dysplasias.
In conclusion we presented two families with overlapping forms of ectodermal dysplasia, VDS and RHS, with mutations in IRF-6 and P63 respectively. The fact that p63 and IRF-6 belong to the same signaling pathways explains the overlapping clinical features among both conditions. Interestingly, physicians should remember that these conditions may have incomplete penetrance and clinical variability even among members of the same family and thus we cannot predict disease severity in subsequent generations.
Conflicts of Interest
None.
Funding
None
Disclosure Statement
Consent for publication was obtained from patients, approved by the Institutional Review Board of the American University of Beirut.
Article Info
Article Type
Case ReportPublication history
Received: Fri 15, Nov 2019Accepted: Thu 19, Dec 2019
Published: Tue 31, Dec 2019
Copyright
© 2023 Mazen Kurban. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ORD.2019.01.04
Author Info
Edgar Jabbour Lamiaa Hamie Mazen Kurban Pamela Kassabian
Corresponding Author
Mazen KurbanDepartment of Dermatology, American University of Beirut Medical Center, Beirut, Lebanon
Figures & Tables
References
- Wenger T, Li D, Harr MH, Tan WH, Pellegrino R et al. (2018) Expanding the phenotypic spectrum of TP63-related disorders including the first set of monozygotic twins. Am J Med Genet A 176: 75-81. [Crossref]
- Deshmukh PK, Deshmukh K, Mangalgi A, Patil S, Hugar D et al. (2014) Van der woude syndrome with short review of the literature. Case Rep Dent 2014: 871460 [Crossref]
- Thomason HA, Zhou H, Kouwenhoven EN, Dotto G-P, Restivo G et al. (2010) Cooperation between the transcription factors p63 and IRF6 is essential to prevent cleft palate in mice. J Clin Invest 120: 1561-1569. [Crossref]
- Gritli-Linde A (2010) p63 and IRF6: brothers in arms against cleft palate. J Clin Invest 120: 1386-1389. [Crossref]
- Ural A, Bilgen F, Cakmakli S, Bekerecioglu M (2019) Van der Woude Syndrome with a Novel Mutation in the IRF6 Gene. J Craniofac Surg. [Crossref]
