Neoadjuvant Target Therapy with Lenvatinib Followed by Reoperative Central Neck Surgery for Thyroid Cancer
A B S T R A C T
Thyroid cancer is the most common endocrine malignancy, with differentiated thyroid cancers, including papillary and follicular cancers, accounting for nearly 95% of cases. These cancers generally have an excellent prognosis. However, RAI (radioiodine)-refractory disease remains a significant challenge for clinicians. Lenvatinib, a potent multikinase inhibitor targeting vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor alpha, and RET and KIT proto-oncogenes, offers a potential treatment option. In this report, we present a case where lenvatinib was utilized in a neoadjuvant setting. The patient had a history of thyroidectomy and neck dissection. Follow-up revealed elevated thyroglobulin (Tg) levels, and physical examination and ultrasonography (US) identified lymphadenopathies in the central neck and strap muscles. Lenvatinib treatment resulted in a 50% reduction in lymphadenopathy size, but was temporarily discontinued due to hypertension, the most common adverse effect. Subsequently, all metastatic lymph nodes and involved strap muscles were successfully resected, with an uneventful postoperative period and no wound complications. As lenvatinib is a relatively new drug, data on its neoadjuvant use is limited.
Keywords
Thyroid cancer, kinase inhibitors, RAI-refractory, lenvatinib, neoadjuvant therapy
Introduction
Thyroid cancers are the leading endocrine malignancies, and differentiated thyroid cancers, comprising papillary and follicular types, generally have favorable outcomes [1]. The incidence of thyroid cancer has been increasing globally, which underscores the need for effective treatment strategies. Surgery and RAI therapy are effective treatments for differentiated thyroid cancers [2]. However, RAI-refractory thyroid cancers present ongoing challenges for clinicians, as they do not respond to standard radioactive iodine treatment. Chemotherapy and external beam radiotherapy (EBRT) can be used for unresectable tumors, but EBRT-induced tissue changes complicate subsequent surgical interventions. Additionally, these traditional therapies often come with significant side effects that can impact the patient’s quality of life. As a result, there is a critical need for alternative therapies that are both effective and have a manageable safety profile [3].
Kinase inhibitors, such as lenvatinib, offer a promising alternative for RAI-refractory patients as neoadjuvant therapy. Lenvatinib has shown efficacy in reducing tumor size and delaying disease progression, making it a valuable tool in the management of aggressive thyroid cancers. This report discusses a case in which lenvatinib was used to downsize the tumor, facilitating successful surgical resection of all metastatic lymph nodes and involved tissues [3].
Case Description
A 50-year-old male presented with a lump in the neck. Physical examination identified a nodule in the left thyroid lobe, and laboratory tests, including thyroid function tests, were within normal ranges. Preoperative fine-needle aspiration biopsy was consistent with oncytic neoplasm of the thyroid. The patient underwent bilateral total thyroidectomy, and the pathology report revealed a 4 cm hurthle cell carcinoma in the left lobe with evidence of vascular invasion but no lymphatic invasion (Figure 1).
Figure 1: Thyroidectomy specimen.
Post-surgery, the patient received 150 mCi RAI, which is a standard dose for ablating any remaining thyroid tissue or microscopic disease. Follow-up scintigraphy showed no residual disease, indicating an initial successful response to treatment. However, during subsequent follow-ups, a rise in Tg levels was detected, suggesting possible recurrence or metastasis.
Further imaging with PET/CT scans revealed metastases in the lateral neck (level 3), prompting a central and lateral neck dissection. Histopathological examination of the resected tissues showed that only one of six central lymph nodes was metastatic (1 mm metastasis size), and none of the 42 lateral neck lymph nodes were metastatic. Oncocytic carcinoma tumor deposits showing infiltration in a multinodular pattern, with diameters of 26 mm, 12 mm, 11 mm, and 10 mm, was observed within the connective tissue.This finding indicated a relatively limited metastatic spread at that stage (Figure 2).
Figure 2: Lateral neck disection specimen.
In late 2023, during a routine follow-up, multiple palpable tumors were noted on physical examination, and neck ultrasonography confirmed the presence of suspicious nodules. Comprehensive mutation profiling was performed on the tumor specimen, including analysis of MYC, PTEN, TP53, POLD1, MSI, TMB, LOH, and PD-L1 expression. Based on these results, no mutations were detected for targetable therapy. Given the limited treatment options available, lenvatinib was ultimately initiated. Lenvatinib was chosen due to its targeted mechanism of action and previous reports of efficacy in similar cases.
The patient started on lenvatinib at a standard dose, and within a few weeks, a significant reduction in the size of the tumors was observed (Figures 3 & 4). Specifically, a 50% decrease in the size of the tumors was noted, which is a considerable response given the refractory nature of the disease. However, treatment was temporarily discontinued due to the development of hypertension, the most common adverse effect associated with lenvatinib. Hypertension management included antihypertensive medications and close monitoring of blood pressure.
Figure 3: US of one of the tumors before levatinib treatment.
Figure 4: US showing the decrease in size of the same tumor in (Figure 3).
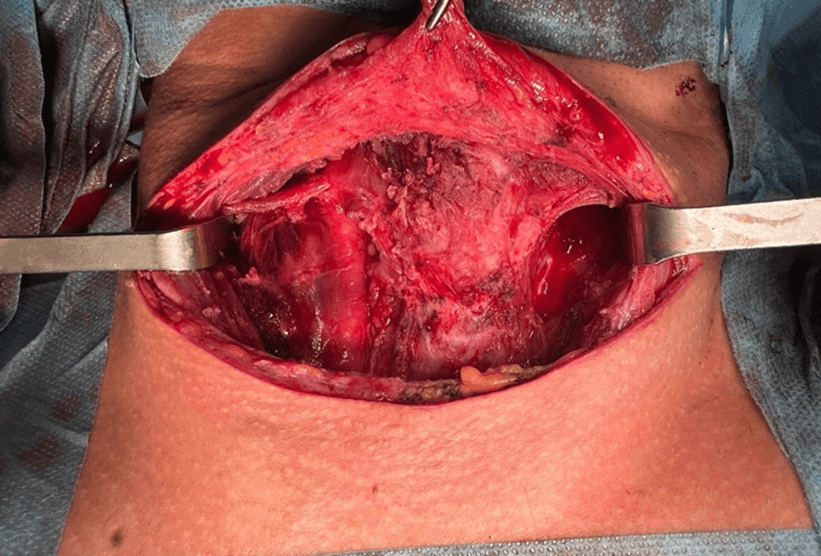
After stabilization of the patient's blood pressure, another neck dissection was performed to remove all tumors located in level 6, right levels 3 and 6, and within the strap muscles. Histopathological examination of the resected tissues showed that none of the lymph nodes from the right lateral neck were metastatic, although other resected nodules were confirmed to be metastatic. Multiple isolated oncocytic-type carcinoma metastases were detected under the skin and within the muscle tissue. The postoperative period was uneventful, with no wound complications or other significant adverse events due to lenvatinib (Figures 5 & 6).
Figure 5: Neck dissection specimen.
Figure 6: Picture of the neck after dissection.
Discussion
Thyroid cancer is the most common endocrine malignancy, with differentiated thyroid cancers (papillary and follicular) comprising 95% of cases. These cancers typically have an excellent prognosis, with distant metastases occurring in less than 10% of patients and a mortality rate of 0.5 deaths per 100,000 individuals. The primary treatment for thyroid cancer is surgery, which may suffice for many patients with localized disease [4].
However, more aggressive forms of thyroid cancer require additional treatments, including RAI therapy, which is effective in treating disseminated disease. The problem arises with RAI-refractory disease or locally invasive cancers involving critical structures such as the trachea, esophagus, or jugular vein. These cases may necessitate extensive resections with significant morbidity and mortality risks, or they may render the cancer unresectable [5 ,6].
The introduction of kinase inhibitors like lenvatinib has revolutionized the treatment landscape for RAI-refractory thyroid cancer [7]. Lenvatinib is a targeted multikinase inhibitor approved for the treatment of RAI-refractory thyroid cancer. It targets multiple receptors and proto-oncogenes involved in tumor growth and angiogenesis, including vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor alpha, and RET and KIT proto-oncogenes [3, 8].
The efficacy of lenvatinib in locally recurrent or progressive RAI-refractory thyroid cancer was demonstrated in the pivotal study of lenvatinib in differentiated cancer of the thyroid (SELECT). This phase III, randomized, double-blind study compared lenvatinib to placebo and showed significant benefits in progression-free survival, leading to the approval of the drug. Lenvatinib is administered orally, making it a convenient option for patients [4].
Common adverse effects of lenvatinib include hypertension, proteinuria, diarrhea, loss of appetite, and weight loss, while rare adverse effects include fistula formation. Dose adjustments or temporary discontinuation of the drug may be necessary to manage these adverse effects. Antiangiogenic agents like lenvatinib are known to impair wound healing due to their effects on blood vessel formation. In the SELECT trial, a 7-day withdrawal period before surgical intervention was recommended to prevent wound complications [2, 4, 9].
Despite the generally favorable prognosis for differentiated thyroid cancers, the 10-year survival rate among patients with differentiated thyroid cancer that is refractory to radioiodine (iodine-131) therapy is only 10% from the time of detection of metastasis [4, 8]. This stark statistic underscores the critical need for effective treatments like lenvatinib to improve outcomes for these patients.
In our case, lenvatinib treatment resulted in a significant reduction in the size of metastatic tumor deposits, facilitating successful surgical resection. The patient's postoperative recovery was uneventful, with no wound healing issues, likely due to the careful management of lenvatinib discontinuation before surgery. This case highlights the potential of lenvatinib as a neoadjuvant therapy to downsize tumors, making them more amenable to surgical intervention.
Given the relatively recent approval of lenvatinib, there is still much to learn about its optimal use in different clinical scenarios. Future studies should focus on identifying the most effective dosing regimens, managing adverse effects, and understanding the long-term outcomes of patients treated with lenvatinib. Additionally, combining lenvatinib with other therapeutic modalities, such as immunotherapy, may offer new avenues for enhancing its efficacy and improving patient outcomes.
Conclusion
Lenvatinib represents a promising treatment option for patients with RAI-refractory thyroid cancer, particularly in the neoadjuvant setting. Our case demonstrates the efficacy of lenvatinib in reducing tumor size and facilitating surgical resection, even in challenging cases with limited treatment options. Careful management of adverse effects, particularly hypertension, is crucial for maximizing the benefits of lenvatinib. As lenvatinib continues to be integrated into clinical practice, ongoing research and clinical experience will help refine its use and improve outcomes for patients with thyroid cancer.
Data Availability Statement
Not applicable.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 07, Oct 2024Accepted: Sat 19, Oct 2024
Published: Thu 31, Oct 2024
Copyright
© 2023 Hakan Kaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2024.03.01
Author Info
Hakan Kaya Elif Peker Erol Metecan Düren
Corresponding Author
Hakan KayaDepartment of Endocrine Surgery, Acıbadem Maslak Hospital, Istanbul, Turkey
Figures & Tables





References
1. Zafón C, Castelo B
(2022) Use of multikinase inhibitors/lenvatinib in singular thyroid cancer
scenarios. Cancer Med 11 Suppl 1: 3-4. [Crossref]
2. Brose MS,
Panaseykin Y, Konda B, De La Fouchardiere C, Hughes BGM et al. (2022) A
Randomized Study of Lenvatinib 18 mg vs 24 mg in Patients With
Radioiodine-Refractory Differentiated Thyroid Cancer. J Clin Endocrinol
Metab 107: 776-787. [Crossref]
3. Cabanillas ME,
Habra MA (2016) Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer
Treat Rev 42: 47-55. [Crossref]
4. Wirth LJ, Durante
C, Topliss DJ, Winquist E, Robenshtok E et al. (2022) Lenvatinib for the
Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer: Treatment
Optimization for Maximum Clinical Benefit. Oncologist 27: 565-572. [Crossref]
5. Alshehri K,
Alqurashi Y, Merdad M, Samargandy S, Daghistani R et al. (2022) Neoadjuvant
lenvatinib for inoperable thyroid cancer: A case report and literature review. Cancer
Rep (Hoboken) 5: e1466. [Crossref]
6. Dang RP, McFarland
D, Le VH, Camille N, Miles BA et al. (2016) Neoadjuvant Therapy in
Differentiated Thyroid Cancer. Int J Surg Oncol 2016: 3743420. [Crossref]
7. Iwasaki H, Toda S,
Ito H, Nemoto D, Murayama D et al. (2020) A Case of Unresectable Papillary
Thyroid Carcinoma Treated with Lenvatinib as Neoadjuvant Chemotherapy. Case
Rep Endocrinol 2020: 6438352. [Crossref]
8. Schlumberger M,
Tahara M, Wirth LJ, Robinson B, Brose MS et al. (2015) Lenvatinib versus
Placebo in Radioiodine-Refractory Thyroid Cancer. N Engl J Med 372:
621-630. [Crossref]
9. Tsuboi M, Takizawa H, Aoyama M, Tangoku A (2017) Surgical treatment of locally advanced papillary thyroid carcinoma after response to lenvatinib: A case report. Int J Surg Case Rep 41: 89-92. [Crossref]
