Journals
Neurologic Wilson Disease Presenting with Acute Delirium And Automatic Writing Behaviour: A Case Report
A B S T R A C T
Introduction: Wilson’s disease (WD) is a rare autosomal recessive disorder which leads to abnormal copper deposition in multiple tissues. Due to extensive deposition of copper in the liver and brain, WD primarily manifests with a wide range of symptoms as well as psychiatric symptoms.
Method: We report a 15-year-old boy presenting with automatic writing behavior and acute neuropsychiatric symptoms; splenomegaly and elevated transaminase levels.
Result: A diagnosis of WD was later made, following liver biopsy. Previously, increased writing activity has been reported in cerebrovascular disease, frontal lobe dementia, temporal lobe epilepsy, Parkinson disease and multiple sclerosis but not in WD.
Conclusion: This is the first report of increased writing behavior in WD.
Keywords
Wilson's disease, delirium, automatic writing behavior
Introduction
Wilson’s disease (WD) is an autosomal recessively inherited disease causing abnormal copper deposition in multiple tissues [1]. WD occurs due to mutations of the ATP7B gene on chromosome 13, which encodes a metal-transporting P-type ATPase (ATP7B). Over 500 mutations of the ATP7B gene has been identified so far, and is reported that 380 mutations have a confirmed role in the pathogenesis of the disease [2]. The average prevalence of WD in the world is estimated as 30 per million with a gene frequency of 1 in 90-150 [3, 4].
Due to extensive deposition of copper in the liver and brain, WD primarily manifests with a wide range of symptoms involving several organ system as well as psychiatric symptoms. Various other organ systems such as cornea, reproductive system, bones, joints, heart, pancreas, skin and endocrine system may also be involved [3, 5]. Kayser-Fleischer ring represent copper deposition in the descemet’s membrane of the cornea [6]. Although Kayser-Fleischer ring is considered to be specific for neurological involvement of the disease, only 77.8- 85.25% of WD patients with neurological involvement have been reported to have Kayser-Fleischer ring [7].
Neurologic symptoms secondary to cupper deposition usually present in the second or third decade of life [8]. The challenging part of neuro-Wilson’s disease is the fact that most of the symptoms are subtle and progress slowly; therefore its diagnosis can easily be overlooked. Most of the patients presenting with neurologic symptoms are usually evaluated for either neurological or psychiatric disorders before they are diagnosed with WD. In this case report, we report a 15-year-old boy presenting with acute neuropsychiatric symptoms who was later had the diagnosis of Wilson’s disease.
Case report
A 15-year-old male patient was referred to our emergency room with symptoms of insomnia, hallucinations, personality changes, aggressive behavior and talking with meaningless words for the last 7 days. He had been evaluated by a pediatric psychiatrist in the emergency room, and was diagnosed as being in delirium and was referred to pediatric neurology for investigation of an underlying organic pathology. The patient’s previous medical history was uneventful. When seen in the emergency room, the patient was conscious but disoriented, very agitated, giving inappropriate answers to questions and talking to himself. His vital signs were normal. He had a sardonic expression produced by dystonic spasm of the fascial muscles on his face and, could understand most simple orders but would resist obeying them.
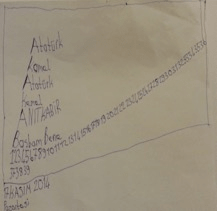
Neurological examination revealed exaggerated deep tendon reflexes, tandem gait ataxia, resting tremor in his hands and impaired writing. Upon hospital admittance, it was noted that the patient had an urge to write all the time, but his writing was unorganized. He started writing from any part of the paper and the content of his writing was meaningless mostly including private names or repetition of adjective. He would appear restless when pen and paper were taken and stopped writing only when he was asleep (Figure 2). Physical examination revealed splenomegaly approximately 3 cm below the left costal margin. His liver was nonpalpable and his sclera was subicteric. There was consanguinity among the parents, and the patient had 3 healthy male siblings. During history taking, no history of drug abuse or an acute infection in preceding weeks was obtained. There was not any chronic neurological disease in the family and first-degree relatives. Although previous developmental and medical histories of the patient were obscure, academic performance of the patient was reported less than moderate when compared to his siblings, and he discontinued his education after the 8th grade.
Figure 1: The patient's handwriting before treatement
Figure 2: The patient's handwriting after treatement
In the laboratory findings; complete blood count revealed pancytopenia with hemoglobin 11 g/dL, total leukocyte count of 3800/mm³ and platelets 118.000/mm³. Serum electrolytes and renal functions were within normal limits. Total serum bilirubin was 1.8 mg/dL (direct bilirubin 1.4 mg/dL) showing dlrect hyperbilirubinemia. Serum aspartat aminotransferase (AST) was 117 IU/L, alanin aminotransferase (ALT) 123 IU/L, alkaline phosphatase (ALP) 253 IU/L, and GGT (gamma-glutamyl transferase) was 334 IU/L. Prothrombin time (PT), activated partial thromboplastin time (aPTT), serum total protein and albumin levels were within normal ranges. Viral serology was negative. Serum alpha-1 antitrypsin was normal and anti nuclear antibody (ANA), anti smooth muscle antibody (ASMA) and liver kidney microsomal antibody (LKM) were negative. Although serum ceruloplasmin level was normal (0.3 g/L, normal range: 0.2–0.6 g/L), his 24 hours urine copper excretion was slightly increased (55 μg/day, normal range: < 40 μg/day). Kayser Fleischer ring was not detected on ophthalmoscopy examination. Ultrasonographic evaluation of abdomen revealed splenomegaly and a normal hepatic size and homogeneous parenchymal echogenicity, while the doppler ultrasonography discovered a dilatated portal vein (a diameter of 13 mm) without any portosistemic collateral formation or ascites. Cranial MRI imaging demonstrated hyperintensities on T1 weighed sequences, and hypointensities on T2 and flair sequences of basal ganglia without any contrast uptake (Figure 1, 2).
Histopathological examination of the liver biopsy revealed biliary type piece meal necrosis in the liver paranchyme without any steatosis. There was fibrous expansion in the portal areas as well as porto-portal bridging fibrosis. The orcein stain did not show any copper accumulation in hepatocytes. Hepatic copper content was 755 μg/g dry weight (normal range: <50 μg/g). The patient was diagnosed with Wilson's disease and trientine chelation with zinc therapy was initiated. During follow-up, liver function tests, 24-hour urine copper excretion and serum ceruloplasmin levels were performed. Although compliance to the treatment and follow-up visits was poor, his insistent writing behavior disappeared during the first week of treatment (F. At the end of the first year, liver function tests and urine copper excretion were within normal limits, and his ataxia, mood and psychiatric complaints improved, while minimal static tremor in his hands continued.
Discussion
Wilson's disease is a genetic disorder in which copper builds up in the body, mainly in the liver and brain. Based on the diagnostic criteria of American Association for the Study of Liver Diseases, WD can be diagnosed by the combination of Kayser Fleischer rings, low serum ceruloplasmin level and high 24-hour urinary copper excretion [3]. If these three criteria are not fully met, a liver biopsy for measurement of copper in dry liver tissue and histopathological examination and/or molecular testing should be performed to confirm the diagnosis. In our patient, neither KF was present nor serum ceruloplasmin level was lower than normal. Apart from acute neuropsychiatric symptoms, diagnosis of WD in our case was based on increased urinary copper excretion and hepatic copper content, which remains the best biochemical evidence for WD.
The clinical manifestations of WD are predominantly hepatic, neurologic, and psychiatric with many patients having a combination of symptoms. There is a wide variability of clinical manifestations at the time of presentation. Regardless of the clinical symptoms present initially, patients often develop other clinical manifestations particularly neurologic symptoms as the disease progresses. Neurological manifestations of WD are numerous, and have been classified as akinetic-rigid syndrome similar to Parkinson disease (reported in 19-62% of WD patients); pseudosclerosis (tremor +/- dysartria) (22-55% of WD patients); ataxia and dystonic syndrome (seen in 11-65% of WD patients) [8]. As the disease progresses, single neurological symptom may develop into a combination of complex neurological signs and symptoms with different level of severity [3]. Neurological signs and symptoms of WD are dysarthria, gait disturbances, ataxia, dystonia, tremor, pseudobulbar palsy, seizures, migraine headaches and insomnia [1]. Dysarthria is the most common neurologic symptom in WD with a given incidence of 85-97% of patients with neuro-Wilson [9]. About one third of patients initially present with psychiatric symptoms like declining school performance, personality changes, labile mood, inappropriate behavior, and in later ages psychotic features resembling paranoia, schizophrenia or depression [4]. The initial symptoms may be misdiagnosed as behavioral problems of puberty. At presentation, acute neuropsychiatric manifestations of our patient looked like delirium. Neuropsychiatric manifestations of WD may mimic acute encephalopathy, which could be misdiagnosed as delirium. Since psychiatric symptoms are often ill defined and attributed to other causes, diagnosis of WD may be difficult during period in which psychiatric symptoms are the sole manifestation, and it may impede diagnosis and treatment [9].
Neurologic symptoms of our case could not correspond to usual neurological manifestations of WD, and were discrete. Careful history taking revealed that our patient had subtle neurological and psychiatric symptoms like labile mood and declining school performance long before the presentation. Increased liver enzymes and presence of splenomegaly and icterus were leading factors for the diagnosis of underlying pathology of acute neuropsychiatric presentation in our patient.
automatic writing behavior and hypergraphia. It has been suggested that the cause and site of lesions responsible for each is completely different [10]. In automatic writing behavior, the content of the writing may be more substantive, and described as loose and devoid of meaning. In most of reported cases, it was a transient finding and has been shown to last for a couple of weeks after an acute neurological insult. Radiological and neuropsychological studies have shown involvement of frontal lobes but the mechanism still remains obscure. By contrast, in hypergraphia, there is meticulous, detailed and compulsive writing, which mostly include religious issues. Hypergraphia has mostly been described in temporal lobe lesions like temporal lobe epilepsy or right-sided brain lesions. Increased writing activity has been reported in cerebrovascular disease, frontal lobe dementia, temporal lobe epilepsy, Parkinson disease and multiple sclerosis but to our knowledge not in WD. In a previously reported case who had automatic writing behavior due to left inferior capsular genu infarction, an interruption of cortico-basal ganglia-thalamocortical loop was shown by SPECT images [11]. Brain pathology in WD can be widespread and include the thalamus, subthalamic nuclei, brainstem, and frontal cortex. Grossly, the brain often demonstrates atrophy and increased ventricular size. Cavitation and cyst formation in the putamen and frontal lobes is seen in advanced disease. Spongy degeneration of the cereberal cortex and subcortical white matter, particularly in the frontal lobes might occur [9]. The most common initial MR imaging abnormality among patients with WD is the occurrence of high T1 signal intensity in the globus pallidus, putamen, and mesencephalon in association with hepatic dysfunction or high T2 signal intensity in the striatum among patients with neurologic symptoms [12]. In our patient MRI images showed abnormalities in basal ganglia and midbrain but not in cortical structures. Although not specific for WD, these abnormalities together with the patient’s clinical findings supported the diagnosis of WD.
To our knowledge, this is the first case to report increased writing behavior in WD. Although there is no cortical involvement in WD, these symptoms may have arisen due to interruption of cortico-basal connection. The fact that this symptom disappeared after treatment and appeared during discontinuation of therapy supports this observation.
Conclusion
Wilson disease when diagnosed late or untreated may progresses to hepatic failure or severe neurologic disability and death, while those treated adequately might have normal life span with no morbidity 9. It is important to be suspicious of WD in any young adolescents presenting with unexplained acute neurological or psychiatric findings accompanied by asymptomatic chronic liver disease. Because severe neurologic findings are usually only partially treatable with medication, and may lead to high morbidity, early diagnosis and compliance with treatment is important.
Conflict of interest
The authors declare no conflict of interest.
Article Info
Article Type
Case ReportPublication history
Received: Sat 04, May 2019Accepted: Fri 21, Jun 2019
Published: Sat 17, Aug 2019
Copyright
© 2023 Deniz Ertem. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JBEM.2019.01.01
Author Info
Deniz Ertem Dilşat Türkdoğan Engin Tutar Esra Polat Gazanfer Ekinci Gülten Thomas
Corresponding Author
Deniz ErtemDivision of Pediatric Gastroenterology, Marmara University School of Medicine, Istanbul, Turkey
Figures & Tables

References
- Aftab A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (2007) Wilson’s disease. Lancet 369: 397-408. [Crossref]
- Wilson disease mutation database.
- Roberts EA, Schilsky ML (2008) Diagnosis and treatment of Wilson disease: an update. Hepatology 47: 2089-2111. [Crossref]
- European Association for Study Liver (2012) EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol 56: 671-685. [Crossref]
- Kumar MK, Kumar V, Singh PK (2013) Wilson’s disease with a neurological presentation, without hepatic involvement in two siblings. J Clin Diagn Res 7: 1476-1478. [Crossref]
- Wadera S, Magid MS, McOmber M, Carpentieri D, Miloh T (2011) Atypical presentation of Wilson disease. Semin Liver Dis 31: 319-326. [Crossref]
- Youn J, Kim JS, Kim HT, Lee JY, Lee PH et al. (2012) Characteristics of neurological Wilson's disease without Kayser-Fleischer ring. J Neurol Sci 323: 183-186. [Crossref]
- Dalvi A (2014) Wilson's disease: neurologic and psychiatric manifestations. Dis Mon 60: 460-464. [Crossref]
- Lorincz MT (2010) Neurologic Wilson’s disease. Ann N Y Acad Sci 1184: 173-187. [Crossref]
- Britton TC (1997) Increased writing activity in neurological disease. Lancet 349: 372-373. [Crossref]
- Suzuki K, Miyamoto T, Miyamoto M, Hirata K (2009) Transient Automatic Writing Behavior following a Left Inferior Capsular Genu Infarction. Case Rep Neurol 1: 8-14. [Crossref]
- Kim TJ, Kim IO, Kimb WS, Cheon JE, Moon SG et al. (2006) MR imaging of the brain in Wilson Disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol 27: 1373-1378. [Crossref]
