Orofacial Myofunctional Therapy with and without Photobiomodulation in the Rehabilitation of Radiation-Induced Trismus: Case Series
A B S T R A C T
Photobiomodulation (PBM) as a therapeutic technology is justified by the biochemical changes caused in the intracellular environment, such as increased production of adenosine triphosphate and activation of antioxidant enzymes, allowing early recovery and maintenance of the homeostasis and proper functioning. This case report aimed to describe the effect of orofacial myofunctional therapy associated or not with photobiomodulation in the rehabilitation of radio-induced trismus in 6 patients. Two intervention modalities were performed, with three patients undergoing OMT isolated and the other two subjects undergoing oral myofunctional therapy associated with photobiomodulation therapy (OMT+PBM). All participants completed the radiotherapy between 3 and 15 months before starting the trismus rehabilitation. The mouth opening was 21.00mm for the patients who underwent exclusive OMT and reached 30.25mm at the end of the rehabilitation (difference of 9.25mm), but for the other three patients submitted to OMT+PBM, it went from 8.4mm to 31.5mm (difference of 23,1mm). It was observed that patients who performed PBM+OMT had greater tolerance to the protocol exercises and less pain report. OMT+PBM was a good combination for trismus rehabilitation and could be considered in further randomized clinical trials.
Keywords
Head and neck neoplasms, masseter muscle spasm, exercise therapy, radiation-induced fibrosis
Introduction
Head and neck neoplasms can have adverse effects on swallowing, chewing, breathing and speech, and can deteriorate quality of life of patients [1]. Radiotherapy can be started with the curative objective, adjuvant (pre or postoperatively) or as palliative treatment, but the side effects that occur when normal cells located within the treatment area suffer temporary or permanent damage. The sequelae resulting from this treatment depend on the number of doses and intensity of exposure and may manifest during or after treatment conclusion [2]. Among the most prevalent complications is trismus, defined as a restriction in mouth opening that can be caused by tumor infiltration into the masticatory muscles and/or the temporomandibular joint; by radiotherapy itself when it involves these muscles in the radiation field; or even a combination of both [3].
Radio-induced trismus originates from fibrosis in the masticatory muscles, which, when affected by irradiation, initially reacts through an abnormal proliferation of fibroblasts, accentuating the synthesis of collagen that leads to the formation of thick fibrous tissue [4]. The diagnostic criterion for trismus is usually defined by mouth openings smaller than 35 millimeters (mm) [5-7]. The subjective diagnosis, based on the patient's clinical complaint such as locking, difficulty opening the mouth, and muscle stiffening should be considered; however, it is less reliable from a scientific point of view, so measurement using a caliper is indicated.
Some therapy options have been used in the rehabilitation of radio-induced trismus, including photobiomodulation therapy (PBM), also known as LASER therapy or low-level LASER therapy. It is a therapeutic approach that modulates biological activity through the use of light in red and infrared wavelengths, which causes positive therapeutic results, including a significant reduction of inflammatory processes, pain relief, prevention of fibrosis and improvement in wound healing and tissue regeneration [8-10].
This technique can be used alone or associated with orofacial myofunctional therapy seeking a significant gain in strength, considerably reducing levels of muscle fatigue, thus optimizing the performance of musculoskeletal functions related to speech; dysphagia, through improvements in saliva flow; improvements in chewing and swallowing functions; muscle toning or relaxation; modulation of inflammation in the treatment of facial paralysis; stimulation and regeneration of the injured nerve and post-surgical recovery, providing faster reduction of edema [11]. This case report was conducted to describe the effect of conventional speech therapy with and without PBM to aid in the rehabilitation of radio-induced trismus.
Case Presentation
This study describes the interventions performed in six patients diagnosed with severe radio-induced trismus. Study was approved by the Research Ethics Committee under opinion number 5.106.387. All participants signed an informed consent form. Prior to the interventions, all participants underwent clinical anamnesis, mouth opening measurement, visual analogue scale (VAS) and Gothenburg Trismus Questionnaire (GTQ). The classification regarding the mouth opening measure was according to the severity of restriction being considered trismus values equal to or less than 35 mm [5-7].
All participants were instructed to remain with their necks in a neutral position and to open their mouths as wide as possible, avoiding excessive pain. A 6 inches (150mm) stainless steel digital caliper was used, from the Stainless Hardened brand. Three measurements were taken and the largest was considered. The GTQ is used as a screening to measure the impact of trismus on aspects related to dietary limitations, fatigue and muscle tension, as well as problems related to the jaw. In each domain, the research subject answered the questions by marking the most convenient answer qualitatively (e.g., responding about the existence of jaw fatigue as “not at all”, “mild”, “moderate”, “severe”, “very severe”) and scores were assigned to the items in such a way that a higher score meant worse performance in quality of life with regard to trismus [12]. The VAS aimed to subjectively measure the perception of pain experienced by patients before and after therapeutic procedures. Patients should answer about their degree of pain, with “0” meaning total absence of pain and “10” the maximum level of pain bearable by the subject.
Interventions
Two intervention modalities were performed: 1) Oral Myofunctional Therapy (OMT) for three patients and OMT plus photobiomodulation therapy (OMT+PBM) for the other three patients. The OMT consists of three mandibular mobility exercises and two traction exercises that should be performed three times a day, daily for five weeks [13]. In addition to performing the OMT programme for mouth opening at home, the participants underwent two weekly sessions in the clinic, supervised by the speech therapist, lasting 30 minutes each, for adjustments, monitoring of the evolution and clinical conditions.
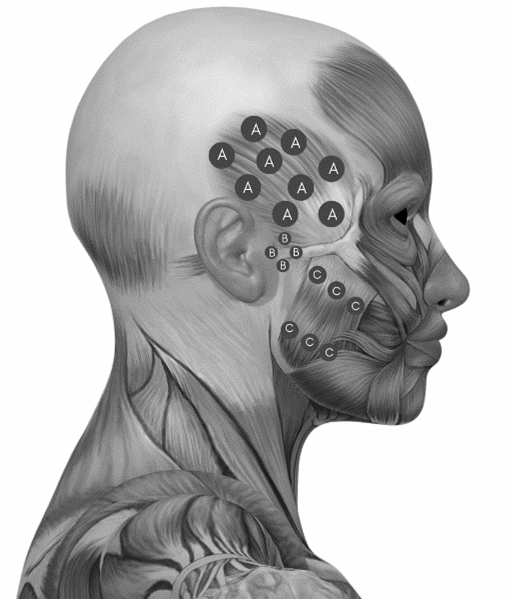
The PBM was conducted with 100 Watts (W) of power and an output spot with an area of 3.3 mm2 (MMO® Brand, LASER duo model). The dosimetric parameters were: red wavelength (660nm) and infrared (808nm) separately irradiated, energy of 6 joules (J) per point, fluence of 199.98 J/cm2, type of continuous emission, which were applied for 60 seconds in each point [10]. The application was punctual, with contact, extra-oral, bilaterally on the temporal muscle (9 points), temporomandibular joint (TMJ) (4 points) and masseter muscle (6 points) (Figure 1). Participants were also instructed to perform OMT for mouth opening three times a day throughout all treatment period (five weeks). After the end of the protocol, all patients were reassessed and followed up in outpatient speech therapy.
Figure 1: PBM application points. A) Temporal muscle. B) Temporomandibular joint (TMJ). C) Masseter muscle.
Source: Adapted from a picture available on google images.
The final sample consisted of six participants, all male, aged between 53 and 76 years, who completed the radiotherapy treatment between 3 and 15 months before the start of the speech therapy intervention for trismus. Below are the sociodemographic data of the participants (Table 1).
Table 1: Sociodemographic data of the participants.
|
|
OMT |
PBM
+ OMT |
||||
|
Patients |
P1 |
P2
|
P3
|
P4 |
P5
|
P6
|
|
Sex |
M |
M |
M |
M |
F |
M |
|
Age |
76 |
64 |
52 |
56 |
33 |
57 |
|
Diagnosis |
HPV-related
oropharyngeal SCC |
Oral
cavity SCC |
Oropharyngeal SCC |
Oropharyngeal SCC |
Oral
cavity SCC |
Oropharyngeal SCC |
|
Cancer
Staging |
T4N2M0 |
pT4pN0M0 |
T4acN1M0 |
T4acN2Mx
|
T1cN0M0-I |
pT2pN2bM0 |
|
Time
after RT (months) |
3 |
32 |
5 |
12 |
6 |
6 |
|
Method of RT |
2D |
2D |
2D |
2D |
2D |
2D |
|
Dose of RT |
70
Gy |
70
Gy |
60
Gy |
70
Gy |
60
Gy |
70
Gy |
|
Intervenção
realizada |
OMT |
OMT |
OMT |
PBM+OMT |
PBM+OMT |
PBM+OMT |
Table 2 shows the results of the pre- and post-intervention mouth opening measurement, as well as the reassessment performed three months after the end of the protocol. Regarding the mouth opening measure, all participants had pre-intervention values below normal parameters. After ten sessions of orofacial myofunctional therapy alone (OMT) and photobiomodulation therapy associated with the conventional modality (PBM+OMT), all participants reached values above the normal level (35mm) [5-7].
Table 2: Mouth opening measurement (mm).
|
Patients |
Before intervention |
After intervention |
Follow-up |
Diference* |
|
P1 - OMT |
26.4 |
44 |
48.2 |
17.6 |
|
P2 - OMT |
15.6 |
16.5 |
16.8 |
1.2 |
|
P3 - OMT |
25.5 |
26.2 |
27 |
1.5 |
|
P4 - PBM+ OMT |
11.5 |
36.6 |
42.2 |
30.7 |
|
P5 - PBM+ OMT |
5.3 |
25.5 |
24.5 |
19.2 |
|
P6 - PBM+ OMT |
15.2 |
43.2 |
46.5 |
31.3 |
Mm:
Millimeters; OMT: Orofacial Myofunctional Therapy isolated; PBM+OMT:
Photobiomodulation associated with Orofacial Myofunctional Therapy; P: Patient (1,
2, 3, 4, 5, 6). *Difference between the pre-intervention and after 3 months of
Follow-Up.
There was total pain reduction in patients who received OMT+PBM. Patients who received only OMT reported mild pain after the end of the intervention, which remained until the third month of follow-up (Table 3).
Table 3: Pain
evaluation (VAS).
|
Patient |
Before
intervention |
After
intervention |
Follow-up
(3 months) |
|
P1
- OMT |
9 |
3 |
2 |
|
P2
- OMT |
9 |
9 |
9 |
|
P3
- OMT |
8 |
2 |
2 |
|
P4
- PBM+ OMT |
8 |
0 |
0 |
|
P5
- PBM+ OMT |
10 |
0 |
0 |
|
P6
- PBM+ OMT |
10 |
2 |
0 |
Mm:
Millimeters; OMT: Orofacial Myofunctional Therapy isolated; PBM+OMT:
Photobiomodulation associated with Orofacial Myofunctional Therapy; P: Patient
(1, 2, 3, 4, 5, 6). *Difference between the pre-intervention and after 3 months
of Follow-Up.
Regarding the level of referred pain, a total reduction was observed in three patients three months after the end of the PBM protocol associated with orofacial myofunctional therapy (PBM+OMT). The other three patients who received only orofacial myofunctional therapy (OMT) reported mild pain after the end of the intervention, which remained after three months. Table 3 shows the data referring to the level of pain reported by the patients, before and after the end of the interventions and after the 3 months reassessment.
Table 4: Association between trismus and quality of life.
|
Patient |
Before
intervention |
After
intervention |
Follow-up
(3 months) |
|
P1
- OMT |
51 |
6 |
1 |
|
P2
- OMT |
50 |
20 |
29 |
|
P3
- OMT |
52 |
40 |
39 |
|
P4
- PBM+ OMT |
57 |
10 |
9 |
|
P5
- PBM+ OMT |
53 |
20 |
0 |
|
P6
- PBM+ OMT |
60 |
10 |
9 |
OMT:
Orofacial Myofunctional Therapy isolated; PBM+OMT: Photobiomodulation
associated with Orofacial Myofunctional Therapy; P: Patient (1, 2, 3, 4, 5, 6).
Table 4 shows the results of the quality-of-life questionnaire for patients with trismus (Gothenburg Trismus Questionnaire - GTQ). Before the interventions, all patients had significant impairment, with scores between 51 and 60 in the domains related to mouth opening, jaw-related problems, eating limitations, fatigue and orofacial muscle tension. Three months after the end of the treatment protocols, the subjects showed a reduction in the domains evaluated by the GTQ.
Discussion
These cases report the possibility of using PBM in the rehabilitation of trismus when associated with OMT. It was possible to assume that the PBM promotes a synergic effect to the exercises therapy. Probably, mouth opening recovery can benefit from this association, and the long-term effects of combination therapy indicate that PBM may also be acting in the prevention of radio-induced fibrosis.
In view of the damage to the masticatory muscles caused in these patients, mainly by radio-induced fibrosis, it is essential to carefully evaluate the moment of initiation of the intervention as well as to use assertive strategies aimed at maintaining homeostasis and muscle functionality. In recent years, research using electrostimulation and PBM have emerged in order to add to therapeutic programmes aimed at the rehabilitation of trismus.
The intervention protocol proposed in this case series was tolerated by the participants with no expression of unwanted side effects or discomfort. On the contrary, a reduction in pain was observed, especially in patients undergoing PBM associated with OMT. Another factor that allows us to reflect is the fact that fatigue was reduced in participants who underwent PBM, this factor can influence the patient’s adherence to therapy, possibly the use of this resource allows greater acceptability and tolerance of OMT. As for the time after the end of RT, the cases had a variability of 3 to 32 months and, despite including patients with chronic adverse effects, an increase in mouth opening was observed both in those who underwent OMT only and those who had the association of PBM. However, considering the potential for maintaining muscle homeostasis promoted by myofunctional exercise and PBM, it is understood that the sooner the intervention is started, the more promising the results can be.
Radio-induced trismus originates from fibrosis in the masticatory muscles, when located in the radiation area, causing reduced mandibular mobility. Radio-induced fibrosis is a major challenge in rehabilitation as it can last for years after the end of treatment. This is due to the persistence of the alteration, chronic local inflammation that can be characterized by the presence of activated T cells and macrophages that produce various chemical mediators of inflammation, such as prostaglandins (PGs), interleukin-6 (IL-6), Tumor Necrosis Factor (TNF), interferon alpha (IF-ά), transforming growth factor beta (TGF-b) and connective tissue growth factor (CTGF/CCN2) [14, 15].
During fibrogenesis, TGF-b is the main cytokine responsible for the increased production and decreased collagen degradation that occurs after radiotherapy. CCN2 is a multifunctional heparin-binding glycoprotein that is expressed at low levels in normal tissues but overexpressed in fibrotic tissues. This overexpression of CCN2 has been associated with fibrosis in various tissues such as skin and muscle [16].
One study compared therapy through exercises associated with ultrasound and low-intensity PBM and isolated OMT, a configuration similar to the proposal of this series of cases where three patients performed the exercise program exclusively and three the exercises associated with the application of PBM. In synergy with the results of Elgohary et al., it was possible to observe a significant benefit of LASER associated with mouth opening exercises compared to exercise therapy alone [17]. These results deserve an in-depth look as it can be an interesting strategy for the rehabilitation process of these patients and in a shorter period of time.
One of the factors that influence the analysis of training efficiency in this population is patient adherence to training, and this control is understood as arduous and complex in most Randomized Clinical Trials (RCTs). Our study analysed adherence through a form that the patient filled in every time he performed the scheduled exercise. This remote control allows us to evaluate with greater precision the efficiency of the strategies and the viability of the disposition of the number of exercises and daily series [17].
A systematic review analysed proposed strategies for trismus in patients with head and neck cancer in RCTs and identified similarity in benefit of proposed interventions despite high variability in exercise planning. In addition, the assessment of the quality of evidence and the risk of bias provided important information about the heterogeneity of the studies and reinforced the need to evaluate the association of therapeutic resources such as PBM with exercises and to follow a methodological rigor that makes it possible to propose protocols. Likewise, analysing and understanding the biochemical effect of PBM and the best way to use this resource is a determining factor of its effectiveness [18].
The effect of light is photochemical and not thermal and, for this reason, light triggers biochemical changes within cells, which can be compared to the process of photosynthesis in plants, where photons are absorbed by cellular chromophores, triggering chemical changes. Low energy density is offered, but high enough for the target cell to use it in a way that stimulates its membrane and/or organelles. Thus, the cell is induced to biomodulation, that is, it will seek to reestablish the state of normality in the affected region [19].
Although many studies show that PBM acts in the effective modification of biological functions, the complex mechanism with which this resource exerts its therapeutic effects has not yet been fully understood, since its effectiveness may vary according to the different states of the affected tissue, cell type, inferred irradiation parameters, among other factors [20]. The most acceptable theory is that the enzyme cytochrome c oxidase (CcO), released by red and infrared light, acts mainly to increase the production of adenosine triphosphate (ATP), the energy required for cellular functioning, causing a short burst of reactive oxygen species (ROS), which also acts as an antioxidant, helping the body's homeostasis [21].
When PBM is applied to target tissues, it involves four different types of interactions which are reflection, transmission, scattering and absorption. The wavelength is the main factor that determines the penetration depth and energy absorption of the LASER in the tissue. LASERs have precise specificity for tissue components known as chromophores that absorb light of specific wavelengths. The primary chromophores in intraoral soft tissue are melanin, haemoglobin and water, and in hard tissues they are water (H2O) and hydroxyapatite (HA). In general, longer wavelengths will have greater affinity for H2O and HA, while shorter wavelengths are absorbed by pigmented tissue and blood elements [22].
There is evidence on the effect of infrared PBM for the treatment of trismus, but our proposal in this case series analysed the effect of red and infrared PBM. It is possible to infer from the results identified that associating the two modalities can promote superior results in mouth opening. Low power PBM plays a preventive role during radiotherapy and may promote a decrease in the risk of trismus, and a protective effect for muscles, ligaments and other tissues associated with stomatognathic functions. The anti-inflammatory effect and analgesic properties of photobiomodulation therapy together with the stimulation of tissue repair and cell proliferation are the main mechanisms associated with its therapeutic effects.
I Effect of LASER on Skeletal Muscle
The best results observed in patients undergoing low-power LASER, in relation to mouth opening, can be attributed to the regeneration of skeletal muscles, through the activation of quiescent satellite cells, leading to their proliferation and, consequently, reduction of the inflammatory process. Another important point is the ability to reduce oxidative stress, a factor related to degenerative changes such as the development of fibrosis. Regarding cellular mechanisms, the effect of the technique directly impacted the capacity of the mitochondrial respiratory chain, leading to greater production of adenosine triphosphate (ATP) and culminating in higher energy levels [23, 24].
The level of muscle fatigue tends to be influenced by LASER due to the increase in the microcirculation of the irradiated anatomical region, which allows the re-establishment of muscle resistance, which may explain the greater tolerance of P4, P5 and P6 (PBM+OMT group) to exercise protocol. As for fatigue, P2, which only after orofacial myofunctional therapy obtained a score of 29 in the GTQ, indicating that higher hand muscle levels end fatigue three months of treatment [12]. Considering the value equal to or greater than 35 mm as a criterion of normality, patients with PBM therapy reached levels above the established standard after the intervention. The mean mouth opening in millimeters in patients P1, P2 and P3 increased from 26.4, 15.6 and 25.5 mm to 48.2 mm, 16.8 and 27mm (difference of 17.6, 1.2, 1.5 mm), while P4, P5 and P6, submitted to the association of PBM, went from 11.5, 5.3 and 15.2mm to 42.2, 24.5 and 46.5mm (difference of 30.7, 19.2 and 31.3mm). The results obtained and described in this case series demonstrate that photobiomodulation therapy brings benefits to the treatment of radioinduced trismus when associated with OMT, which contrasts with the data reported by Serique et al. where smaller gains were observed with the use of exclusive photobiomodulation [25].
LASER has already demonstrated its ability to optimize muscle function under hypoxic conditions such as mechanical stress, fatigue and neurogenic inflammation, which are responsible for electrolyte and metabolic changes present in patients with radio-induced trismus. These effects are thought to be primarily due to mitochondrial activation, resulting in an increase in electron transport, cellular respiration, oxygen consumption, and ATP production. In addition, it initiates signaling pathways that lead to the activation of various transcription factors and modulate the levels of cytokines, growth factors, and inflammatory mediators. Direct effects on somatosensory and/or motor nerves also participate in muscle relaxation and analgesia induced by laser therapy through neural blockade of nociceptors and inhibition of motor nerves [26-28].
II Effect of LASER on Pain Relief
The effectiveness of low-level LASER in the treatment of pain originating from soft tissue trauma, including those related to radiotherapy, can be attributed to the indirect reduction of edema, bleeding, neutrophilic activity, provocative cytokines and enzymatic action. This treatment modality reduces the characteristic pain observed more markedly in the three cases undergoing photobiomodulation therapy in this series, resulting in better tissue repair, as lymphatic vessel regeneration is accelerated [29, 30].
Literary data validate the effectiveness of photobiomodulation in the management of pain, acute or chronic, related to the impairment of the muscles involved in mouth opening, leading to a reduction in the need for analgesic drugs and maintenance of low pain symptoms in the long term. The analgesic action is related, especially, to the endorphin release that occurs during the systemic process of the LASER in the tissues. Our study observed a reduction in pain levels in all cases, but more markedly in those submitted to the association of exercises with low-frequency LASER. Other studies carried out in similar populations obtained very similar numerical results, with an average reduction of 8 points on the Visual Analogue Scale after the LASER protocol [17, 31].
It is concluded that orofacial myofunctional therapy associated with photobiomodulation promoted greater gains in mouth opening when compared to cases in which OMT was performed isolated. More studies are needed, including a larger sample in an RCT to strengthen this hypothesis.
Conflicts of Interest
None.
Funding
Study funded by the authors.
Article Info
Article Type
Case Series and Review of the LiteraturePublication history
Received: Mon 22, Aug 2022Accepted: Thu 08, Sep 2022
Published: Thu 29, Sep 2022
Copyright
© 2023 Émille Dalbem Paim. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2022.03.02
Author Info
Émille Dalbem Paim Felipe de Oliveira Goulart Vera Beatris Martins Fernanda Tormen Korspalski Fabrício Edler Macagnan
Corresponding Author
Émille Dalbem PaimSpeech Pathologist at Irmandade Santa Casa de Misericórdia de Porto Alegre, Brazil
Figures & Tables
Table 1: Sociodemographic data of the participants.
|
|
OMT |
PBM
+ OMT |
||||
|
Patients |
P1 |
P2
|
P3
|
P4 |
P5
|
P6
|
|
Sex |
M |
M |
M |
M |
F |
M |
|
Age |
76 |
64 |
52 |
56 |
33 |
57 |
|
Diagnosis |
HPV-related
oropharyngeal SCC |
Oral
cavity SCC |
Oropharyngeal SCC |
Oropharyngeal SCC |
Oral
cavity SCC |
Oropharyngeal SCC |
|
Cancer
Staging |
T4N2M0 |
pT4pN0M0 |
T4acN1M0 |
T4acN2Mx
|
T1cN0M0-I |
pT2pN2bM0 |
|
Time
after RT (months) |
3 |
32 |
5 |
12 |
6 |
6 |
|
Method of RT |
2D |
2D |
2D |
2D |
2D |
2D |
|
Dose of RT |
70
Gy |
70
Gy |
60
Gy |
70
Gy |
60
Gy |
70
Gy |
|
Intervenção
realizada |
OMT |
OMT |
OMT |
PBM+OMT |
PBM+OMT |
PBM+OMT |
Table 2: Mouth opening measurement (mm).
|
Patients |
Before intervention |
After intervention |
Follow-up |
Diference* |
|
P1 - OMT |
26.4 |
44 |
48.2 |
17.6 |
|
P2 - OMT |
15.6 |
16.5 |
16.8 |
1.2 |
|
P3 - OMT |
25.5 |
26.2 |
27 |
1.5 |
|
P4 - PBM+ OMT |
11.5 |
36.6 |
42.2 |
30.7 |
|
P5 - PBM+ OMT |
5.3 |
25.5 |
24.5 |
19.2 |
|
P6 - PBM+ OMT |
15.2 |
43.2 |
46.5 |
31.3 |
Mm:
Millimeters; OMT: Orofacial Myofunctional Therapy isolated; PBM+OMT:
Photobiomodulation associated with Orofacial Myofunctional Therapy; P: Patient (1,
2, 3, 4, 5, 6). *Difference between the pre-intervention and after 3 months of
Follow-Up.
Table 3: Pain
evaluation (VAS).
|
Patient |
Before
intervention |
After
intervention |
Follow-up
(3 months) |
|
P1
- OMT |
9 |
3 |
2 |
|
P2
- OMT |
9 |
9 |
9 |
|
P3
- OMT |
8 |
2 |
2 |
|
P4
- PBM+ OMT |
8 |
0 |
0 |
|
P5
- PBM+ OMT |
10 |
0 |
0 |
|
P6
- PBM+ OMT |
10 |
2 |
0 |
Mm:
Millimeters; OMT: Orofacial Myofunctional Therapy isolated; PBM+OMT:
Photobiomodulation associated with Orofacial Myofunctional Therapy; P: Patient
(1, 2, 3, 4, 5, 6). *Difference between the pre-intervention and after 3 months
of Follow-Up.
Table 4: Association between trismus and quality of life.
|
Patient |
Before
intervention |
After
intervention |
Follow-up
(3 months) |
|
P1
- OMT |
51 |
6 |
1 |
|
P2
- OMT |
50 |
20 |
29 |
|
P3
- OMT |
52 |
40 |
39 |
|
P4
- PBM+ OMT |
57 |
10 |
9 |
|
P5
- PBM+ OMT |
53 |
20 |
0 |
|
P6
- PBM+ OMT |
60 |
10 |
9 |
OMT:
Orofacial Myofunctional Therapy isolated; PBM+OMT: Photobiomodulation
associated with Orofacial Myofunctional Therapy; P: Patient (1, 2, 3, 4, 5, 6).
Source: Adapted from a picture available on google images.
References
1.
Santos LL, Teixeira LM
(2011) Oncologia Oral. Lisboa:
Lidel 232.
2.
Rodriguez CGB, de Paula
Eduardo C de P, Aranha ACC, de Freitas PM (2019) Photobiomodulation with
Low-Level LASER in the Treatment of Trismus After Radiotherapy: A Case Report. Photobiomodul Photomed Laser Surg
37: 240-243. [Crossref]
3.
Buglione M, Cavagnini R,
Di Rosario F, Maddalo M, Vassalli L et al. (2016) Oral toxicity management in
head and neck cancer patients treated with chemotherapy and radiation:
Xerostomia and trismus (Part 2). Literature review and consensus statement. Crit Rev Oncol Hematol 102: 47-54. [Crossref]
4.
Teguh DN, Levendag PC,
Voet P, van der Est H, Noever I et al. (2008) Trismus in patients with
oropharyngeal cancer: relationship with dose in structures of mastication
apparatus. Head Neck 30:
622-630. [Crossref]
5.
Dijkstra PU, Huisman PM, Roodenburg JLN (2006) Criteria for
trismus in head and neck oncology. Int
J Oral Maxillofac Surg 35:
337-342. [Crossref]
6.
Scott B, Butterworth C,
Lowe D, Rogers SN (2008) Factors
associated with restricted mouth opening and its relationship to health-related
quality of life in patients attending a maxillofacial oncology clinic. Oral Oncol 44: 430-438. [Crossref]
7.
Ordahan B, Karahan AY
(2017) Role of low-level LASER therapy added to facial expression exercises in
patients with idiopathic facial (Bell’s) palsy. Lasers Med Sci 32: 931-936. [Crossref]
8.
Salazar M, Victorino FR,
Paranhos LR, Ricci ID, Gaeti WP et al. (2008) Efeitos e tratamento da
radioterapia de cabeça e pescoço de interesse ao cirurgião dentista: Revisão da
literatura. Rev Odonto
16: 62-68.
9.
Zecha JAEM, Raber
Durlacher JE, Nair RG, Epstein JB, Elad S et al. (2016) Low-level laser therapy/photobiomodulation
in the management of side effects of chemoradiation therapy in head and neck
cancer: part 2: proposed applications and treatment protocols. Support Care Cancer 24: 2793-2805.
[Crossref]
10. MMO. LASER DUO - Instruções de Uso. 2016: 20.
11. Johnson J, Carlsson S, Johansson M, Pauli
N, Rydén A et al. (2012) Development and validation of the Gothenburg Trismus
Questionnaire (GTQ). Oral Oncol
48: 730-736. [Crossref]
12. Marrafon CS, Matos LL, Simões Zenari M,
Cernea CR, Nemr K (2018) Programa terapêutico fonoaudiológico para abertura de
boca em pacientes com câncer de boca e orofaringe em radioterapia adjuvante:
estudo piloto. CoDAS 30: e20160221. [Crossref]
13. Alterio D, Marvaso G, Ferrari A, Volpe S,
Orecchia R et al. (2019) Modern radiotherapy for head and neck cancer. Semin
Oncol 46: 233-245. [Crossref]
14. Gao Y, Ling T, Wu H (1997) Expression of
transforming growth factor beta 1 in keratinocytes of oral submucous fibrosis
tissue. Zhonghua kou Qiang yi Xue Za Zhi 32: 239-241. [Crossref]
15. Shi Wen X, Leask A, Abraham D (2008)
Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and
fibrosis. Cytokine Growth Factor Rev
19: 133-144. [Crossref]
16. Elgohary HM, Eladl HM, Soliman AH,
Soliman ES (2018) Effects of Ultrasound, Laser and Exercises on
Temporomandibular Joint Pain and Trismus Following Head and Neck Cancer. Ann Rehabil Med 42: 846-853. [Crossref]
17. Chee S, Byrnes YM, Chorath
KT, Rajasekaran K, Deng J
(2021) Interventions for Trismus in Head and Neck Cancer Patients: A
Systematic Review of Randomized Controlled Trials. Integr Cancer
Ther 20: 15347354211006474. [Crossref]
18. El Mobadder M, Farhat F, Nammour S (2019)
Photobiomodulation Therapy in the Treatment of Chronic Dysphagia Post Hormonal
Therapy in a Breast Cancer Patient. Dent
J (Basel) 7: 53. [Crossref]
19. Kamstra JI, Reintsema H, Roodenburg JLN,
Dijkstra PU (2016) Dynasplint Trismus System exercises for trismus secondary to
head and neck cancer: a prospective explorative study. Support Care Cancer 24: 3315-3323. [Crossref]
20. Alan H, Yolcu Ü, Koparal M, Özgür C,
Öztürk SA et al. (2016) Evaluation of the effects of the low-level laser
therapy on swelling, pain, and trismus after removal of impacted lower third
molar. Head Face Med 12:
25. [Crossref]
21. Verma SK, Maheshwari S, Singh RK,
Chaudhari PK (2012) Laser in dentistry: An innovative tool in modern dental
practice. Natl J Maxillofac Surg
3: 124-132. [Crossref]
22. Luo L, Sun Z, Zhang L, Li
X, Yu Dong et al.
(2013) Effects of low-level
LASER therapy on ROS homeostasis and expression of IGF-1 and
TGF-β1 in skeletal muscle during the repair process. Lasers Med Sci 28: 725-734.
[Crossref]
23. Silveira PCL,
da Silva LA, Pinho CA, De Souza PS, Ronsani MM et al. (2013) Effects of low-level laser therapy (GaAs)
in an animal
model of muscular damage induced
by trauma. Lasers Med Sci
28: 431-436. [Crossref]
24. Serique AVC, Carvalho
JT, Rêgo IAP, Queiroz GS, de
Castro Godinho J et al.
(2021) Laserterapia no tratamento de disfunção temporomandibular, trismo e
xerostomia de paciente oncológico: relato de caso. Revista Eletrônica Acervo Saúde 13: e5129.
25. Chung H, Dai T, Sharma SK,
Huang YY, Carroll JD et al.
(2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40:
516-533. [Crossref]
26. Bjordal JM, Lopes‐Martins RAB, Frigo L (2015) Low
level laser therapy – mechanism of action: Inflammatory process. Lasers in dentistry: Guide for Clinical Practice 27-33.
27. Chow R. Low level laser therapy-mechanism
of action: analgesia. Lasers in Dentistry: Guide for clinical practice. Weinheim: Wiley-Blackwell 205:
34-39.
28. Oberoi S, Zamperlini Netto G, Beyene J,
Treister NS, Sung L (2014) Effect of prophylactic low level laser
therapy on oral mucositis: a systematic review and meta-analysis. PLoS One 9: e107418. [Crossref]
29. Elting LS, Keefe DM, Sonis ST, Garden AS,
Spijkervet FKL et al. (2008)
Patient-reported measurements of oral mucositis in head and neck cancer
patients treated with radiotherapy with or without chemotherapy: demonstration
of increased frequency, severity, resistance to palliation, and impact on quality
of life. Cancer 113: 2704-2713. [Crossref]
30. Klausner G, Bensadoun RJ, Champion A, Benzaquen D, Canova CH et al. (2021) État de l’art de la photobiomodulation dans la prise en charge des effets secondaires de la radiothérapie: indications et niveaux de preuve. Cancer/Radiothérapie 25: 584-592.
31. Klausner G, Bensadoun RJ, Champion A, Benzaquen D, Canova CH et al. (2021) State of art of photobiomodulation in the management of radiotherapy adverse events: Indications and level of evidence. Cancer Radiother 25: 584-592. [Crossref]
