Outbreak of Encephalitis Caused by Human Herpesvirus Type 1 (HSV-1) in NonHuman Captive Primates in São Paulo, SP, Brazil
A B S T R A C T
Human herpesvirus type 1 (HSV-1), a Alphaherpesvirinae, has a broad range of cross-species infectivity with considerable variation in expression and disease severity among different hosts. Infection in humans, results in mild disease characterized by mucocutaneous lesions. In some species of nonhuman primates, however, the infection can be lethal. Transmission occurs through food contaminated by humans with labial herpes, offered to monkeys, constituting an important anthropozoonosis. In this study, we describe the occurrence of two outbreaks of encephalitis caused by HSV-1, occurring in the State of São Paulo, Brazil. The first outbreak affected a Brown howler monkey and three marmosets. The Brown howler monkey had tongue ulcers and enteritis and the marmosets were found dead without presenting clinical symptomatology. The second outbreak affected ten marmosets that were found dead suddenly in the enclosure without presenting symptoms or clinical signs. Organ fragments were processed for transmission electron microscopy, histopathology and inoculation in cell culture techniques. Ballooning degeneration, foci of monolymphocytic inflammatory reaction, corpuscle of eosinophilic inclusion, monolymphocytic meningitis and desquamative monolymphocytic enterocolitis, were the main lesions observed by histopathology. By the negative staining technique, enveloped and non-enveloped, herpesvirus particles were found, measuring 120-200 nm in diameter in all samples of organ fragments. The presence of aggregates formed by antigen-antibody complex, characterized the positive result obtained in the immunoelectron microscopy technique for HSV-1. Using the immunocytochemistry technique, the antigen-antibody interaction was strongly enhanced by the colloidal gold particles over the herpesvirus, confirming the presence of HSV-1. Intranuclear incomplete particles measuring 80-100 nm in diameter and complete or enveloped scattered in the cytoplasm, were visualized in ultrathin sections of liver and cell culture measuring 140 nm of diameter. Immature particles budding from cell membranes were also observed. In viral isolation in VERO cells, typical cytopathic effect was observed in brain samples.
Keywords
Human herpesvirus type 1, non-human primates, transmission electron microscopy, histopathology, cell culture
Introduction
Human herpesvirus type 1 (HSV-1), is a member of the Herpesviridae family, Alphaherpesvirinae subfamily and Simplex virus genus [1]. The HSV virions consist of four components, a core containing the virus DNA, an icosahedral capsid surrounding the core, the tegument that surrounds the capsid, and outer lipid bilayer envelope showing spikes on its surface. The isometric enveloped particles measure 120-200 nm of diameter [2]. The viral genome is composed of a linear double-stranded DNA molecule of 152 kbp in length and a GC content of 68% [3]. HSV-1 is neurotropic, has a short replication cycle and is rapidly disseminated and causes lysis in culture of infected cells. Has a broad range of cross-species infectivity with considerable variation in expression and disease severity among different hosts [4, 5]. HSV-1 establishes lifelong infection in the neurons of sensory and autonomic ganglia [6, 7]. Infection in humans, the natural host, most commonly results in mild disease, characterized by recurrent mucocutaneous lesions, such as herpes labialis, genital herpes, herpetic whitlow and keratitis [5]. Sporadic viral encephalitis is one of the complications following primary infection, associated with a high mortality rate and neurologic sequelae among survivors [8]. In some species of non-human primates, the infection can be lethal [4]. Transmission occurs by direct contact with mucosal surfaces, wounds, maternal milk or through of the food contaminated by humans with labial herpes, offered to monkeys, constituting an important anthropozoonosis [4, 9].
The disease affects the neurological system of the monkeys, causing prostration, lingual ulcers, and loss of balance, apathy and death [4]. HSV-1 shows 79% nucleotide sequence identity with monkey B virus, a zoonosis that results in encephalitis or neurological problems in humans, with the majority of monkeys being asymptomatic carriers [10]. Outbreaks of the disease have been reported in various parts of the world, as USA; Germany; Austria; Peru; Thailand; China; Japan and Espanha [4, 5, 11-17]. In Brazil, few reports describe the occurrence of HSV-1 in non-human primates, but all affecting marmosets (Callithrix jacchus and Callithrix penicillata) [6, 18-21].
In this study, we describe the occurrence of two outbreaks of encephalitis caused by human herpesvirus type 1, occurring in the State of São Paulo, Brazil, affecting a Brown Howler Monkey (Allouata guariba) and marmosets (Callithrix jacchus, Callithrix penicillata and Callithrix aurita) this latter framed into the threatened category of extinction. Callithrix aurita, endemic to the Atlantic Forest of southeastern Brazil, have suffered drastic population reduction due to the loss and fragmentation of their habitat mainly by competition and hybridization with invasive species [22].
Materials and Methods
I Description of the Outbreaks
The first outbreak was detected in August 2014, in an Ecological Park, in the State of São Paulo, SP, Brazil. The animals were kept in captivity. A Brown Howler Monkey (Aloutta guariba) and 3 marmosets (Callithrix sp., Callithrix penicilatta e Callithrix jacchus were affected. The Brown Howler Monkey had tongue ulcers and enteritis and the marmosets were found dead without presenting clinical symptomatology. Hemorrhagic and congested lungs and brains of the animals were observed at necropsy. The second outbreak, occurred in 2016. The animals were kept in captivity at a breeding located in São José dos Campos, SP, Brazil, with 40 animals. Ten marmosets (Callitrix aurita) were found dead suddenly in the enclosure without presenting symptoms or clinical signs. During the necropsy pulmonary and intestinal congestion was evidenced and the presence of free sero-sanguinolent liquid in the thoracic cavity.
Organ fragments, kept on ice, were sent to the Electron Microscopy Laboratory of Biological Institute, to be processed for transmission electron microscopy by negative staining, immunoelectron microscopy, immunolabeling with colloidal gold particles and resin embedding techniques and tissues fixed in 10% formalin for histopathology (technique routine histological - H & E) and to the Laboratory of Rabies and Viral Encephalitis for inoculation in cell culture.
II Histopathology
i Routine Histological Technique
All organ fragments (liver, brain, kidneys, lung and small intestine) of the animals were fixed in 10% buffered formalin, dehydrated, diaphanized and embedded in paraffin. 5 µm thick sections were stained with hematoxylin and eosin technique.
ii Transmission Electron Microscopy
a Negative Staining Technique (Rapid Preparation)
In this technique, samples of organ fragments of monkeys were suspended in 0.1M phosphate buffer at pH 7.0. Drops of the obtained suspensions were placed in contact with metallic copper grids, previously covered with a film of 5% collodium amyl acetate and stabilized with carbon. Next, the grids were drained with filter paper and negatively stained with 2% ammonium molybdate at pH 5.0 [23-26].
b Immunoelectron Microscopy Technique
In this technique, the copper grids, previously prepared with collodium film and stabilized with carbon, were first incubated with protein A (1ml/ml) placed in contact with the virus-specific antibody. After that, grids were washed with drops of phosphate buffer 0.1M at pH 7.0, incubated with the viral suspension of the all samples of organ fragments, washed with drops of distilled water and negatively stained with 2% ammonium molybdate at pH 5.0 [2, 23, 25, 27, 28].
c Immunolabeling with Colloidal Gold Particles Technique
At the immunolabeling technique with colloidal gold particles for negative staining, the copper grids were placed in contact with viral suspension of the samples of organ fragments and, after removing the excess with filter paper, the same were put on specific primary antibody drops. After further washing in PBS drops, the grids were incubated in protein A drops, in association with 10 nm colloidal gold particles (secondary antibody). Grids were then contrasted with 2% ammonium molybdate at pH 5.0 [29]. All grids submitted to the above reactions were observed in a Philips EM 208 transmission electron microscope, at 80 kV.
d Resin Embedding Technique
Fragments of liver and cell culture were fixed in 2.5% glutaraldehyde in 0.1 M, pH7.0 phosphate buffer and post-fixed in 1% osmium tetroxide in the same buffer. After dehydration in cetonic series, the samples were embedded in Spurr resin [30, 31]. Ultrathin sections were cut on the LKB ultratome and mounted on copper grids. The sections were contrasted with uranyl acetate-lead citrate [32, 33].
e Inoculation in Cell Culture
VERO cell monolayers were inoculated with a 20% brain suspension of all animals for observation of the cytopathic effect.
Results
I Histopathology
i Routine Histological Technique (H&E)
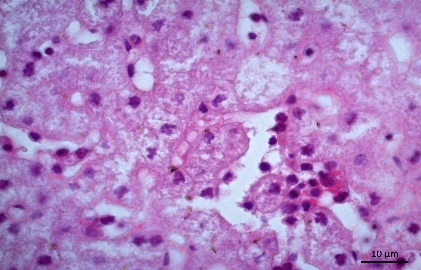
Histopathology revealed that the brain presented gliosis, neuroniophagia, corpuscle of eosinophilic inclusion (Figure 1), marked monolymphocytic meningitis, dilated and congested blood vessels (Figure 2). The liver showed areas of cytolysis in groups of hepatocytes with ballooning degeneration, karyolysis, foci of monolithic inflammatory reaction and necrosis (Figures 3 & 4). The kidneys showed areas of necrosis, marked tubulonefrosis and ballooning degeneration with hyaline material, interstitial hemorrhage with increased Bowman space. Lungs of 2 animals were hemorrhagic. There was also marked desquamative monolymphocytic enterocolitis.
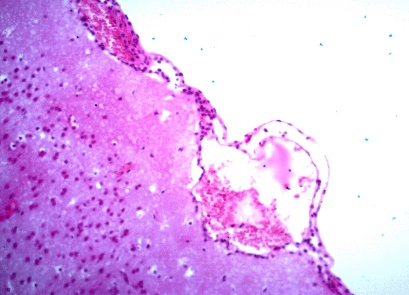
Figure 1: Photomicrography of brain. Corpuscle of eosinophilic inclusion. 630x.
Figure 2: Photomicrography of brain. Monolymphocytic meningitis. Congestion of blood vessels under the meningeal area and gliosis. 200x.
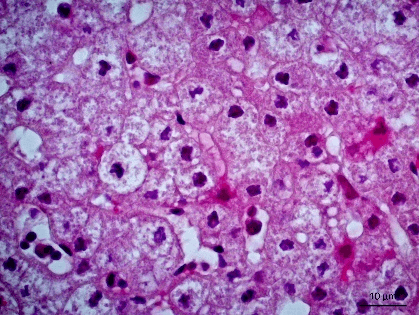
Figure 3: Photomicrography of liver. Areas of cytolysis in groups of hepatocytes with ballooning degeneration. 630x.
Figure 4: Photomicrography of liver. Monolymphocytic hepatitis and ballooning degeneration. 630x.
II Transmission Electron Microscopy
i Negative Staining Technique (Rapid Preparation)
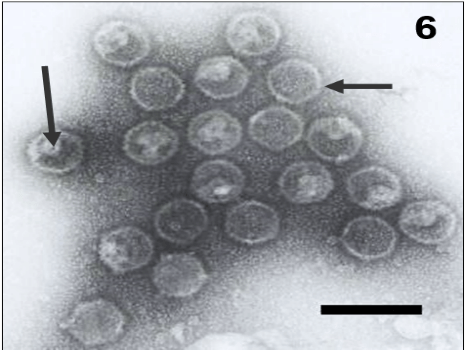
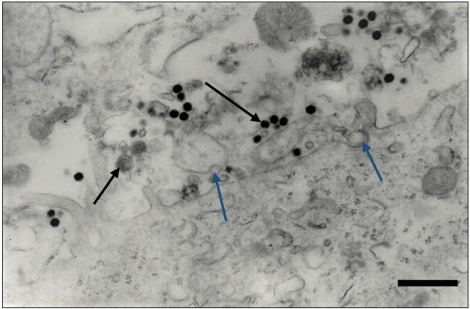
At the transmission electron microscopy all the samples were analyzed by negative staining technique and a great number of particles with similar morphology to isometric herpesvirus exhibits enveloped (Figure 5, arrow) and non-enveloped particles (Figure 6) and showing nucleocapsids stain penetrated (Figure 6, big arrow) and non-stain penetrated (Figure 6, minor arrow), measuring 120-200 nm of diameter was observed.
ii Immunoelectron Microscopy Technique
In all organ fragments samples analyzed, the antigen antibody interaction was characterized by the aggregation of viral particles (Figure 6).
iii Immunolabeling with Colloidal Gold Particles Technique
In this technique, the antigen-antibody interaction was strongly enhanced by the dense colloidal gold particles over the herpesvirus in all samples of organ fragments (Figure 7, arrow), confirming the results of immunoelectron microscopy technique.
iv Resin Embedding Technique
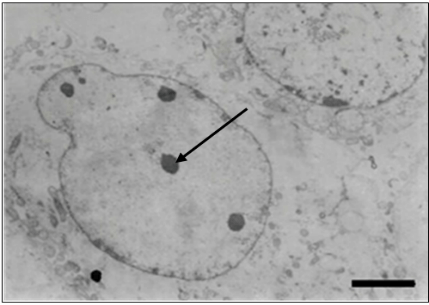
Through the resin embedding technique, hexagonal nucleocapsids (Figures 8 & 9) measuring 80-100 nm in diameter and complete or enveloped particles (Figure 8) scattered throughout the cytoplasm were visualized in ultrathin sections of cell culture and liver of infected animals, measuring 150-170 nm of diameter. Immature particles budding from the cell membrane to form the envelope were also visualized (Figure 8). The presence of empty nuclei containing rounded chromatin masses was also observed (Figure 10).
Figure 5: Negatively stained of enveloped herpes virus particles, showing envelope covered by spikes (arrow). Bar: 85 nm.
Figure 6: In the immunoelectron microscopy technique the type 1 herpesvirus particles were aggregated by antigen-antibody complex. Observe nucleocapsids stain penetrated (big arrow) and non-stain penetrated (minor arrow). Bar: 260 nm.
Figure 7: Antigen-antibody interaction strongly enhanced by the dense gold particles over the type 1 herpesviruses (arrow). Bar: 200 nm.
Figure 8: Ultrathin section of cell culture. Observe: hexagonal nucleocapsids (big arrow), enveloped particles (minor arrow) and immature particles budding from the cell membrane to form the envelope (blue arrow). Bar: 570 nm.
Figure 9: Ultrathin section of cell culture. Hexagonal nucleocapsids scattered throughout the cytoplasm (arrow). Bar: 300 nm.
v Inoculation in Cell Culture
The herpesvirus was isolated in Vero cells in brain samples from the marmosets and the cytopathic effects observed were, cells decrease with rounding, refringence and detachment in 2 days.
Figure 10: Ultrathin section of cell culture. Observe empty nuclei containing rounded chromatin masses (arrow). Bar: 1600 nm.
Discussion
During the occurrence of outbreaks caused by human herpesvirus type 1 in non-human primates of the species Allouata guariba, Callitrix penicillata, Callitrix jacchus and Callitrix aurita, the diagnosis was made through the application of techniques of the histopathology, inoculation in cell culture and transmission electron microscopy. The brown howler monkey (Allouata guariba) had whitish vesicular lesions on the soft palate and ulcerative tongue, as well as enteritis, severe emaciation, prostration and death. Similar symptoms and signs have also been described by other researchers in other species of monkeys infected with HSV-1, Callitrix penicillata; Callitrix jacchus; Aotus nancymae; Callitrix pygmaea; Aotus azarae ; Gorilla beringei graueri; Alouatta caraya; Pithecia pithecia; and Macaca mullata [5, 11-14, 16-18, 20, 21, 34-37].
Other symptoms have also been described, such as, dehydration in Pithecia pithecia and in Hylobates a. agilis, vomiting, serous nasal and ocular discharges in Callitrix jacchus, dyspnea and eye discharge in Callitrix pygmaea , eyebrow, tail and scrotum lesions in Aotus trivigartus, dyscoria in Aotus nancynae, and various neurological signs, such as, convulsion in Pithecia pithecia, Callitrix jacchus, Callitrix penicillata , Hylobates lar and in Aotus trivirgatus, wrist folding and myoclonus in Hylobates a. agilis, hind limb weakness in Aotus trivirgatus, horizontal nystagmus in Alouatta caraya , tic-like symptoms in Callitrix jacchus, disorientation, ataxia and paresis in Hylobates lar, and profuse salivation in Hylobates a. agilis, Calitrix pygmaea, Callitrix jacchus and in the latter associated with aggressive behavior [4, 12-14, 16, 17, 20, 35-39]. Antibodies to HSV-1 were detected in healthy monkeys (gorillas, chimpanzees, orangutans and gibbons) [40, 41].
The marmosets of our study (Callitrix sp, C. penicillata, C. Jacchus and C. aurita) were found dead suddently in the enclosure without presenting symptoms or clinical signs. This profile has also been reported in other studies related to HSV-1, in P. pithecia and in C. jacchus [36, 39]. The histopathological findings that we visualize in the brains of the animals corroborate with those of other reports regarding the presence of gliosis, neuroniophagia, eosinophilic inclusion corpuscle, monolymphocytic meningitis and dilated and congested blood vessels [4, 11, 16, 17, 20, 21, 35-39, 42]. We observed that the kidneys of the monkeys presented areas of necrosis, marked tubulonefrosis and ballooning degeneration with hyaline material, interstitial hemorrhage with increased Bowman space. The kidneys of the A. trivirgatus, C. peniciliata and the A. nancymae, também showed signs of necrosis and inflammation [14, 21, 39]. Casagrande et al. found multinucleated syncytial cells in the kidneys of C. jacchus and C. penicillata [9].
In the liver of all animals we found areas of cytolysis in groups of hepatocytes with ballooning degeneration, caryolysis, foci of monolymphocytic inflammatory reaction and necrosis, histopathological aspects also verified in the liver of the P. pithecia, Callitrix spp., C. jacchus , A. azarae and in the A. trivirgatus [11, 12, 36, 39, 42]. Schrenzel et al. and Kik et al. found that the hepatocytes of the P. pithecia and P. pygmaeus presented numerous eosinophilic intranuclear inclusions [37, 43]. Histological examination of the oral lesion of the G. beringei graueri showed the presence of inclusion bodies and ballooning degeneration [34]. We found that the non-human primates lungs were congested and hemorrhagic, whereas the presence of emphysema alveolar and lymphoplasmacytic inflammatory infiltrate was described in Callitrix spp, C. jacchus and in C. penicillata by Longa et al. and by Casagrande et al., respectively [20, 42].
We also noted the presence of marked desquamative monolymphocytic enterocolitis in the small intestine of the primates of our study, which was also reported in the intestine of Alouatta caraya by Barnes et al., in the intestine and stomach of C. jacchus and C. penicillata by Casagrande et al. and A. azarae by Kreutzer et al. [11, 20, 35]. Fan et al., however, stated that in the structural damage was observed in the brain and other organs of the M. mullata inoculated with HSV-1 [15]. These authors suggested that HSV-1 infection was capable of inducing vesicular lesions in the oral skin or mucosa of mediating viral replication at least in nervous tissues through an unknown mechanism without pathological damage.
The herpesvirus was isolated in Vero cells in brain samples from the marmosets and the cytopathic effects observed were, cells decrease with rounding, refringence and detachment in 2 days, aspects also observed in other studies of HSV-1 in C. jacchus, Hylobates a. agilus and in M. mulatta [13, 15, 38]. To the transmission electron microscope, we visualized by means of negative staining, typical particles of herpesvirus, isometric, exhibits enveloped and non-enveloped particles, showing nucleocapsids stain penetrated and non-strain penetrated, measuring 120-200 nm of diameter. There is no reference in the literature about the use of this technique for the identification of human herpesvirus type 1 in monkeys.
This technique, however, has been widely used to detect herpesvirus particles in other animal species such as bovine, swine, feline and avian [44-50]. The morphological characteristics that we described were also mentioned by these authors. The negative staining technique was also used for the rapid diagnosis of human herpes simplex virus infections and to differentiate the poxviruses from herpesviruses in protocol for rash illness [51, 52]. Likewise, we have also found no reports about the application of the immunoelectron microscopy technique used to demonstrate the presence of aggregates formed by antigen-antibody interaction for type 1 human herpesvirus (HSV-1) in samples from organ fragments of infected monkeys and in cell culture supernatant infected with herpesvirus positive monkey brain.
The immunoelectron microscopy technique was used to agglutinate herpes virus particles in swine semen and in organ fragments of the parrots [49, 53]. This technique was also employed to study the capsids protein (NC1) and (NC2) of the human HSV-1 [54].The Immunolabeling with colloidal gold particles in negative staining technique showed in all samples of the organ fragments and cell culture supernatant extensive marking particles with colloidal gold, obtaining confirmation of the viral strain (HSV-1). Although the technique was not found in the literature to serotype HSV-1 in monkeys, it was used to identify HSV-1 UL56 protein, possibly involved in virus-host interaction, to label conserved epitopes of simian herpesvirus SA8 and bovine herpesvirus type 2, to study morphological aspects of mouse thymic viruses and to differentiate between herpes simplex virus (HSV) and varicella-zoster (VZV), since viral culture and standard serological tests failed to prove VZV infection [2, 54, 55-58].
In ultrathin sections of liver and cell culture of the infected animals, we visualized the presence of incomplete particles or hexagonal nucleocapsids, measuring 80-100 nm in diameter and complete or enveloped particles scattered throughout the cytoplasm, measuring 140 nm of diameter. This morphological description was also mentioned by other authors in HSV-1 research in the liver of the Pongo p. pygmaeus, Aotus azarae and Pithecia p. pithecia, in the neurons of the Alouatta caraya , in the tongue of the Callithrix jacchus, in the lesions of the Gorilla b. graver and in the lung of the Gorilla g. gorilla [11, 12, 34, 35, 37, 43, 59]. In these ultrathin sections, were visualized incomplete particles budding from nuclear membrane to form the envelope, as described in HSV-1 studies in Pithecia p. pithecia and in Gorilla b. graveri realized by Schrenzel et al. and by Gilardi et al., respectively [33, 36].
The monkeys of our work came from Ecological Parks and the caretakers did not use masks and gloves during feeding and therapeutic procedures.The close contact between nonhuman primates and humans facilitates transmission and the infected animals can spread the infection to other nonhuman primates [4, 11-13, 16, 18, 20, 21, 36, 41, 43]. Visitors, students, trainees or handlers might be able to infect the animals, by transference eaten food into the cage. Infected persons can excrete the virus even in the absence of visible lesions [12]. Due to the severity of the disease, control and prevention measures should be instituted along with the Ecological Parks and breeding, such as the awareness of visitors not to feed the animals during the visitation, orientation of handlers on the need to wear appropriate clothing, gloves and masks, during treatment and feeding of animals, training of good management practices and hygiene in the enclosures, besides the adoption of procedures of quarantine to the new animals [14, 16, 36, 43].
The viral identification through the use of histopathology techniques, inoculation in cell culture and transmission electron microscopy allowed the characterization of encephalitis caused by human herpesvirus type 1 during the occurrence of the outbreak. These results enabled the rapid implementation of prophylactic measures and control of the disease in the Ecological Parks, avoiding the occurrence of new deaths, considering that the Buffy-tuffed-ear marmoset (Callitrix aurita) is a species in the threatened category of extinction by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) list [22].
This report is the first detection of HSV-1 in Callitrix aurita and the first applications of the transmission electron microscopy techniques (negative staining, immunoelectron microscopy and immunolabeling with colloidal gold particles) for detection of type 1 human herpesvirus in nonhuman primates.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 10, Feb 2020Accepted: Fri 28, Feb 2020
Published: Thu 12, Mar 2020
Copyright
© 2023 Marcia Helena Braga Catroxo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2020.01.02
Author Info
Ana Maria Cristina Rebello Pinto da Fonseca Martins Eliana Monteforte CassaroVillalobos Liliane Milanelo Marcia Helena Braga Catroxo Maria do Carmo Custódio de Souza Hunold Lara
Corresponding Author
Marcia Helena Braga CatroxoBiological Institute, São Paulo, SP, Brazil
Figures & Tables




References
- ICTV-2016 – Taxonomy – International Committee on Taxonomy of Viruses.
- Doane FW, Anderson M (1987) Electron microscopy in diagnostic virology – A pratical guide and atlas. Cambridge, MA: Cambridge University Press 178p.
- Pellet ER, Roizman B (2013) Herpesviridae. Knipe DM, Howley PM. Fields Virology, Philadelphia: Wolters Kluwer-Lippincott Williams and Wilkins 1803-1822.
- Landolfi JA, Wellehan JFX, Johnson AJ, Kinsel MJ (2005) Fatal human herpesvirus type 1 infection in a white-handed gibbon (Hylobates lar). J Vet Diagn Invest 17: 369-371.[Crossref]
- Roizman B, Knipe DM, Whitley RJ (2013) Herpes simplex viruses In: Knipe DM, Howley P, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B. Fields Virology. New York, USA: Wolters Kluwer-Lippincott-Williams and Wilkins 1823-1897.
- Lee S, Ives AM, Bertke AS (2015) Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol 89: 8383-8391. [Crossref]
- Smith G (2012) Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol 66: 153-176. [Crossref]
- Prasad A, Lu M, Lukac DM, Zeichner SL (2012) An alternative kaposi's sarcoma-associated herpesvirus replication program triggered by host cell apoptosis. J Virol 86: 4404-4419. [Crossref]
- Tischer BK, Osterrieder N (2010) Herpesviruses - a zoonotic threat? Vet Microbiol 140: 266-270. [Crossref]
- Huff JL, Barry PA (2003) B-Virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerging Infect Dis 9: 246-250. [Crossref]
- Kreutzer R, Kreutzer M, Gunther CP, Matz-Rensing K, Wohlsei P (2011) Systemic herpesvirus infection in an Azara’s Night Monkey (Aotus azarae). J Med Primatol 40: 197-199. [Crossref]
- Matz-Rensing K, Jentsch KD, Rensing S, Langenhuyzen S, Verschoor E et al. (2003) Fatal herpes simplex infection in a group of common marmosets (Callithrix jacchus). Vet Pathol 40: 405-411. [Crossref]
- Huemer HP, Larcher C, Czedik-Eysenberg T, Nowotny N, Reifinger M (2002) Fatal infection of a pet monkey with human herpesvirus 1. Emerg Infect Dis 8: 639-641. [Crossref]
- Gozalo AS, Montoya EJ, Weller RE (2008) Dyscoria associated with herpesvirus infection in owl monkeys (Aotus nancymae). J Am Assoc Lab Animal Sci 47: 68-71. [Crossref]
- Fan S, Cai H, Xu X, Feng M, Wang L et al. (2017) The characteristics of herpes simplex virus type 1 infection in Rhesus macaques and the associated pathological features. Viruses 9: E 26. [Crossref]
- Imura K, Chambers JK, Uchida K, Nomura S, Suzuki S et al. (2014) Herpes simplex virus type 1 infection in two pet marmosets in Japan. J Vet Med Sci 76: 1667-1670. [Crossref]
- Juan-Sallés C, Ramos-Vara JA, Prats N, Solé-Nicolás J, Segalés J et al. (1997) Spontaneous herpes simplex virus infection in common marmosets (Callithrix jacchus). J Vet Diagn Invest 9: 341-345. [Crossref]
- Araújo JL, Frade MTS, Melo CMF, Carneiro RS, Galiza GJN et al. (2016) Infecção sistêmica por herpesvírus simples em um sagui-detufo-branco (Callithrix jacchus) no semiárido da Paraíba. Vet Zootec 23: 203-208.
- Bruno SF, Liebhold MM, Matz-Rensing K, Romão MAP, Didier A et al. (1997) Herpesvirus infection in free-living black-tufted-ear marmoset (Callithrix penicillata E Geoffroyi 1812) at the State Park of Serra da Tiririca, Niterói, Rio de Janeiro, Brazil. Brasilien Berl MunchTierarztl Wochenschr 110: 427-430. [Crossref]
- Casagrande RA, Pannuti CS, Kanamura C, Freire WS, Grespan A et al. (2014) Fatal human herpesvirus 1 (HHV-1) infection in captive marmosets (Callithrix jacchus and Callithrix penicillata) in Brazil: clinical and pathological characterization. Pesq Vet Bras 34: 1109-1114.
- Costa EA, Luppi MM, Malta MCC, Luiz APMF, Araújo MR et al. (2011) Outbreak of human herpesvirus type 1 infection in nonhuman primates (Callithrix penincillata). J Wildl Dis 47: 690-693. [Crossref]
- Barth OM (2011) Contrastação negativa In: Souza, W. Técnicas básicas de Microscopia Eletrônica aplicadas às Ciências Biológicas: Rio de Janeiro, RJ, Brazil, Sociedade Brasileira de Microscopia e Microanálise 87-99.
- Brenner S, Horne RW (1959) A negative staining method for high resolution electron microscopy of viruses. Biochem Biophys Acta 34: 103-110. [Crossref]
- Hayat MA, Miller SE (1990) Negative Staining. New York: Mc Graw-Hill Publ Company 253p.
- Madeley CR (1997) Electron microscopy and virus diagnosis. J Clin Pathol 50: 454-456. [Crossref]
- Berthiaume AR, Malaughlin B, Payment P, Trepainer P (1981) Rapid detection of human viruses in feces by a simple and routine immune electron microscopy technique. J Gen Virol 55: 223-227. [Crossref]
- Katz D, Kohn A (1984) Immunosorbent electron microscopy for detection of viruses. Adv virus Res 29: 169-194. [Crossref]
- Knutton S (1995) Electron microscopical methods in adhesion. Methods Enzymol 253: 145-158. [Crossref]
- Gonzalez-Santander R (1969) Técnicas de microscopia eletrônica en biología. Madrid, SP: Aguilar 666 p.
- Luft JA (1961) Improvements in an epoxy resin embedding methods. J Biophys Biochem Cytol 9: 409-414. [Crossref]
- Reinolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17: 208-212. [Crossref]
- Watson ML (1958) Staining of tissue sections for electron microscopy with heavy metals. J Biophyis Biochem Cytol 4: 475-478. [Crossref]
- Gilardi KVK, Oxford KL, Gardner-Roberts D, Kinami J-F, Spelman L et al. (2014) Human herpes simplex virus type 1 in confiscated gorilla. Emerg Infect Dis 20: 1883-1886. [Crossref]
- Barnes KJ, Garner MM, Wise AG, Persiani M, Maes RK et al. (2016) Herpes simplex encephalitis in a captive black howler monkey (Alouatta caraya). J Vet Diag Invest 28: 76-78. [Crossref]
- Lapid R, Eshar D (2017) Fatal herpes simplex virus 1 (HSV-1) infection in a group of Zoo kept white-faced saki monkeys (Pithecia pithecia) in Israel. Israel J Vet Med 72: 51-55.
- Schrenzel MD, Osborn KG, Shima A, Klieforth RB, Maalouf GA (2003) Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia). J Med Primatol 32: 7-14. [Crossref]
- Mootnick AR, Reingold M, Holshuh HJ, Mirkovic RR (1998) Isolation of a herpes simplex virus type 1-like agent from the brain of a mountain agile gibbon (Hylobates agilis agilis) with encephalitis. J Zoo Wildl Med 29: 61-64. [Crossref]
- Deisboeck TS, Wakimoto H, Nestler U, Louis DN, Sehgal PK et al. (2003) Development of a novel non-human primate model for preclinical gene vector safety studies. Determining the effects of intracerebral HSV-1 inoculation in the common marmoset: a comparative study. Gene Ther 10: 1225-1233. [Crossref]
- Eberle R, Hilliard JK (1989) Serological evidence for variation in the incidence of herpesvirus infections in different species of apes. J Clin Microbiol 27: 1357-1366. [Crossref]
- Sakulwira K, Theamboonlers A, Charoonrut P, Poovorawan Y (2002) Serological evidence of herpesvirus infection in gibbons. BMC Microbiol 2: 11. [Crossref]
- Longa CS, Bruno SF, Pires AR, Romijn PC, Kimura LS et al. (2011) Human herpesvirus 1 in wild marmosets, Brazil, 2008. Emerg Infec Dis 17: 1308-1310. [Crossref]
- Kik MJL, Bos JH, Groen J, Dorrestein GM (2005) Herpes simplex infection in a juvenile orangutan (Pongo pygmaeus pygmaeus). J Zoo Wildl Med 36: 131-134. [Crossref]
- Hoferer M, Braun A, Sting R (2017) Creation of a bovine herpes virus 1 (BoHV-1) quantitative particle standard by transmission electron microscopy and comparison with established standards for use in real time PCR. Biologicals 48: 121-125. [Crossref]
- Kruger ER, Penha TR, Stoffelo DRE, Roehe PM, Ribeiro MC et al. (2015) Bovine herpesvirus 4 in Paraná State, Brazil: case report, viral isolation, and molecular identification. Braz J Microbiol 46: 279-283. [Crossref]
- 45. Martins AMCRPF, Bersano JG, Ogata R, Amante G, Catroxo MHB et al. (2013) Diagnosis to detect porcine transmissible gastroenteritis virus (TGEV) by optical and transmission electron microscopy techniques. Int J Morphol 31: 706-715.
- Scherba G, Hajjar AM, Pernikoff DS, Sundberg JP, Basgall EJ et al. (1988) Comparison of a cheetah herpesvirus isolate to feline herpesvirus type 1. Arch Virol 100: 89-97. [Crossref]
- Sun H, Li Y, Jiao W, Liu C, Liu X et al. (2014) Isolation and identification of feline herpesvirus type 1 from a south China tiger in China. Viruses 6: 1004-1014. [Crossref]
- Catroxo MHB, Martins AMCRPF, Souza F, Nastari BDB (2015) Herpesvirus detection by transmission electron microscopy, during Pacheco's disease outbreak in Blue-fronted Parrot (Amazona aestiva). Proceedings of the 25th Brazilian Congress of Microscopy, Armação dos Búzios, RJ, Brazil, 2015.
- Tomaszewski E, Wilson VG, Wigle WL, Phalen DN (2001) Detection and heterogeneity of herpesviruses causing Pacheco’s Disease in parrots. J Clin Microbiol 39: 533-538. [Crossref]
- Singh A, Preiksaitis J, Romanowski B (2005) The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol 16: 92-98. [Crossref]
- Laue M, Bannert N (2010) Detection limit of negative staining electron microscopy for the diagnosis of bioterrorism related micro-organisms. J Appl Microbiol 109: 1159-1168. [Crossref]
- Pongiluppi T, Bersano JG, Moreno AM, Borges SRT, Petrella S et al. (2007) Pesquisa de agentes virais em sêmen de suínos pelas técnicas de microscopia eletrônica de transmissão. LAES HAES 2: 108-128.
- Vernon SK, Ponce de Leon M, Cohen GH, Eisenberg RJ, Rubin BA (1981) Morphological components of herpes virus. III Localization of herpes simplex virus type 1 nucleocapsid polypeptides by immune electron microscopy. J Gen Virol 54: 39-46. [Crossref]
- Kehm R, Gelderblom HR, Darai G (1998) Identification of the UL56 protein of herpes simplex virus type 1 within the virion by immuno electron microscopy. Virus Genes 17: 49-53. [Crossref]
- Borchers K, Ozel M, Pauli G, Gelderblom HR, Ludwig H (1990) Conserved epitopes of simian herpesvirus SA 8 and bovine herpesvirus type 2. Arch Virol 111: 1-14. [Crossref]
- Athanassious R, Alain R, Lussier G (1990) Electron microscopy of mouse thymic virus. Arch Virol 113: 143-150. [Crossref]
- Folkers E, Vreeswijk J, Wagenaar F, Kapsenberg JG, Hulsebosch HJ et al. (1992) Immunoelectron microscopy for rapid diagnosis of varicella zoster virus in a complicated case of human T-Cell lymphotropic virus type 1 infection. J Clin Microbiol 30: 2487-2491. [Crossref]
- Heldstab A, Ruedi D, Sonnabend W, Deinhardt F (1981) Spontaneous generalized herpesvirus hominis infection in a lowland gorilla (Gorilla gorilla gorilla). J Med Primatol 10: 129-135. [Crossref]
- Melo FR, Ferraz DS, Valença-Montenegro MM, Oliveira LC, Pereira DG et al. (2015) Avaliação do risco de extinção de Callithrix aurita (E Geoffroy, 1812) no Brasil. ICMBio – Instituto Chico Mendes de Conservação da Biodiversidade – Ministério do Meio Ambiente, 7198.
