Ovarian Teratoma in Pediatric: A Case Series
A B S T R A C T
Introduction: Ovarian teratoma is one of the most common tumor types in pediatric ovarian tumor. Ovarian tumor is rare, which constitutes only 1% of all tumors in pediatric patients. Both imaging and laboratory tools are needed and the management in the past decades seem to be giving good outcome.
Case Series: We report 11 cases of ovarian teratoma who came to our institution during the period of 2016-2019.
Conclusion: Proper examinations should be done in diagnosing ovarian teratoma. Surgery (tumor excision along with or without oophorectomy or salpingo-oophorectomy) and chemotherapy (in malignant teratomas) are the standard therapy for ovarian teratoma and appeared to be quite satisfying result.
Keywords
Ovarian teratoma, pediatric
Introduction
Ovarian teratoma is one of the most common tumor types in pediatric ovarian tumor. Ovarian tumor is rare, which constitutes only 1% of all tumors in pediatric patients. About 47-87.7% of this tumor are germ cell tumors in pediatric age, while only 10% are malignant, yet in women, ovarian germ cell tumors account for 20-25% of all ovarian neoplasm, but only 3-5% are malignant. A study in Japan conducted by Oue et al., incidence of mature teratomas was 61% and incidence of malignant tumors was 9.8%. Diagnostic tools needed to diagnose this are blood serum levels of AFP, beta-hCG, and sometimes LDH, Ca-125, Ca 19-9 and CEA, and imaging studies. Oophorectomy followed by chemotherapy (in malignant type) are routinely performed in managing ovarian tumor. Over the past 3 decades, germ cell tumor survival rates have improved with more aggressive surgical staging and combination chemotherapy [1, 2].
In this series of 11 cases, we described the clinical finding, other examination findings, management and outcome of pediatric patients with ovarian teratoma in the last 4 years in Dr. Soetomo General Academic Hospital.
Case Series
Case 1
An 11-year-old girl with a complaint of abdominal mass in the lower left quadrant for 3 months. She also complained of intermittent abdominal tenderness, without any symptoms of bowel obstruction. Her abdominal physical examination showed palpable mass from the infraumbilical region to left lower quadrant sized 10x8x5 cm, solid, flat surface, fixed and tender. Figure 1 shows patient’s abdominal physical examination. Abdominal CT scan (Figure 2) showed heterogenic enhancing solid mass, irregular edge, size of 12.6x7.6x11.8 cm at the abdominal to pelvic cavity. Patient’s AFP, ß-HCG and LDH were 2.1 ng/ml, < 2 mUI/ml, and 826 ng/ml respectively. Tumor excision along with left salpingo-oophorectomy were then performed and the mass was found on the left ovary, well localized without any seeding to surrounding tissues. It was diagnosed as left ovarian teratoma. Pathological examination showed the mass as germ cell tumor which was dysgerminoma. Cisplatin and etoposide were given afterwards. Patient had been regularly followed up in the next 6 months and her abdominal CT showed no mass in the abdominal nor pelvic cavity/ surgical bed with decreased level of AFP, beta-hCG, and LDH.
Figure 1: Patient’s abdominal clinical appearance.
Figure 2: Abdominal CT showed mass with in abdominal and pelvic cavity.
Case 2
7-year-old girl was brought to the pediatric surgery outpatient clinic because her abdomen seemed to be larger in the past 1.5 months before hospital admission. Size of the abdominal mass was approximately 10x7x10 cm on palpation. There was no complaint related to defecation nor urination. Abdominal ultrasound (Figure 3) revealed solid mixed with cystic mass, well-defined, size of 10.9x6.92 cm, round shaped on the left adnexa suggesting ovarian teratoma. The AFP was 0.4 ng/ml and ß-HCG was < 2 mUI/ml. Tumor excision and left salpingo-oophorectomy were also done in this patient and we found mass on the left ovary without any seeding to surrounding tissues. Pathological result of the excised tumor was ovarian cystic mature teratoma (consisting of skin epidermal layer along with the sebaceous gland, hair follicle, fat tissue and bone cartilage). Intraoperative documentation is shown in (Figures 4 & 5). Outcome of this patient after operation has been showing no recurrence.
Figure 3: Abdominal ultrasound showing ovarian mass.
Figure 4: Operative finding in 7-year-old girl with left ovarian teratoma.
Figure 5: Excised ovarian mass.
Case 3
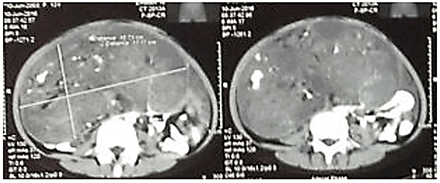
Female, 14-years-old was referred to our hospital being diagnosed with teratoma. She had complained of abdominal mass with abdominal enlargement since the past 2 months until she was admitted to the hospital. She then had abdominal pain and tenderness on her lower abdomen which then spread to the lumbar region. From her physical examination, there was an abdominal distension with palpated mass (20x10x25 cm), solid, ill-defined, and irregular surface. Her abdominal CT (Figure 6) showed abdominal and pelvic cavity mass with a size of 18.7x11.17x25 cm mixed of solid, cystic and calcification suggesting ovarian teratoma with right hydronephrosis. AFP result was high (469.2 ng/ml) and Ca-125 of 459.1 U/ml. Tumor excision with left salpingo-oophorectomy and omentectomy were performed, and the pathological finding revealed that it was immature ovarian teratoma with implantation into the omentum. After surgery, she was given chemotherapy of etoposide, cisplatin and bleomycin. The evaluation result was satisfying with no mass recurrence with AFP level decreased to 26 ng/ml.
Figure 6: Mass in abdominal and pelvic cavity showed in abdominal CT.
Case 4
5-year-old girl with a chief complaint of abdominal mass since 2 years prior to hospital admission. The mass was small which then seemed to get larger as the size of adult’s hand palm. She had been complaining of abdominal pain 2 days before being admitted to the hospital with fatigue and vomiting. Her abdomen was distended and there was palpable mass at the lower to upper right quadrant, mobile ill-defined, and tender. Her serum AFP increased (> 1000 ng/ml), also the LDH level (984 ng/ml), and Ca-125 of 435.5 U/ml, with normal range of ß-HCG. Ultrasound examination, in (Figure 7), revealed solid mass, heterogenic echo parenchymal with a size of 6.36x5.37 cm at the right ovary with ascites. Her AFP result was > 1000 ng/ml. Abdominal CT showed (Figure 8) solid mixed with cystic mass from the ovary with a size of 8.71x7.79 cm, ascites, and right pleural effusion.
Figure 7: Ultrasound revealing solid mass at the right ovary with ascites.
Figure 8: Abdominal CT showing solid mass from the internal genitalia (ovary).
Intraoperative finding showed serous-hemorrhagic peritoneal fluid, right ovarian tumor (8x5x3 cm) with perforated capsule, para-aorta lymph enlargement and omental tumor seeding (Figures 9 & 10). Tumor excision along with right salpingo-oophorectomy and omentectomy were performed and she was given chemotherapy afterwards. Patient’s pathological result was right ovarian tumor suggestive of yolk sac tumor penetrating the capsule with metastasis to the omental and peritoneum. One month postoperative and chemotherapy, another abdominal CT to evaluate the mass was performed and showed no lesion nor mass in the abdominal and pelvic cavity, but there was moderate-severe right hydronephrosis with bilateral pleural effusion and para-aorta lymphadenopathy at the level of L1-L5. She received chemotherapy of bleomycin and etoposide, but then she was then hospitalized because of the kidney failure and severe pleural effusion, but her kidney function status kept decreasing, the chemotherapy was stopped and replaced with palliative care. This patient passed away 6 months after surgery.
Figure 9: Intraoperative finding of right ovarian tumor with omental seeding.
Figure 10: Right ovarian tumor with perforated capsule.
Case 5
An 8-year-old girl with intermittent abdominal pain for 7 days before being admitted to the hospital at the left lower quadrant, especially when palpated. She felt an enlargement of her abdomen, too. In the physical examination, her abdomen was not distended, normal peristaltic sound with palpable mass at the left lower quadrant, size of 8x14x10 cm and tenderness at the site of the mass. There was an increase of AFP (679.4 ng/ml) with normal ß-HCG. Her abdominal CT revealed enhancing solid mixed with cystic mass at the lower abdominal quadrant (probably from the right adnexa) towards pelvic cavity, and lymph node enlargement at the para-aorta, peritumoral, mesenterial and iliac. Tumor excision and left salpingo-oophorectomy were done, and right ovary tumor was found, size of 12x12x10 cm, perforated at the posterior side with intraabdominal spillage, seeding to the mesosigmoideal and omental part. These intraoperative findings can be seen in (Figures 11 & 12). Left ovary and uterus seemed to be normal, and there was no infiltration into the urinary bladder. Pathological examination result was ovarian yolk sac tumor, thereafter she received chemotherapy regimen consisting of cisplatin and etoposide. Her evaluation CT 6 months later showed no mass, and her AFP level decreased to 3.3 ng/ml.
Figure 11: Right ovarian tumor with perforation at the posterior side, seeding into the mesosigmoid and walling off towards the mesosigmoid.
Figure 12: Right ovarian mass after tumor excision.
Case 6
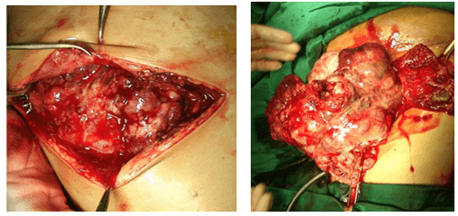
6-year-old patient, female with abdominal enlargement in the past one year with abdominal pain 2 months prior to hospital admission. She also com In physical examination, there was a palpable abdominal mass in the left lower quadrant with diameter size of 10 cm. AFP serum found increased (115.04 ng/ml) with normal ß-HCG. Level of CEA, Ca-125 and Ca-19-9 serum was 2.12 ng/ml, 72.9 ng/ml and 216.26 ng/ml respectively. During the operation, there was found left ovary tumor retroperitoneally with a size of 18x12x15 cm, capsulated, multilobulated attached to the omentum with no seeding. In toto left ovary teratoma excision was performed. Tumor can be seen in (Figures 13 & 14). Pathological result revealed as left ovary mature teratoma. Outcome after surgery a month later showed no recurrence with good clinical condition.
Figure 13: Left ovarian tumor identification, found retroperitoneally, attached to the omentum with mesenterial lymph node enlargement.
Figure 14: Left ovarian rumor (18x12x15 cm).
Case 7
1-year-old female with abdominal mass since 3 months before being admitted to the hospital. The mass had been getting larger with no tenderness. Abdominal solid mass was palpated on on the right illiacal region of abdomen, 17x15 cm. AFP and ß-HCG serum level was normal (0.9 ng/ml) and <2.0 mIU/ml), while Ca-125 was found slightly increased of 38.2 U/mL. Abdominal CT-scan showed cystic capsulated mass with intramural nodule and intracystic calcification in the pelvic cavity to umbilical region, sized 15x10x18.2 cm. During operation, right ovary cystic mass was found, well-defined, gray-white coloured, size of 15x10x15 cm. There was yellow-coloured fluid and hair within the cyst. We performed right salpingo-oophorectomy. Conclusion of pathological examination confirmed this mass as mature ovarian teratoma. After surgery, patient was evaluated in the pediatric surgery outpatient clinic with no complaint of recurrent mass.
Case 8
Female, 12-year-old with a chief complaint of enlarging abdominal mass since 4 months prior to hospital admission. On abdominal inspection, there was bulging mass in all abdominal quadrants and there was a palpable, 20x19 cm solid mass. Patient’s AFP and ß-HCG level was normal 0.7 ng/ml and < 2 mIU/ml). Her CEA was 1.90 ng/ml with increased level of Ca-125 which was 84.6 U/mL. Abdominal CT (Figure 15) showed mass cystic lesion with calcification, sized 12.7x17.5x21.7 cm within the pelvic cavity towards umbilical region.
Figure 15: Abdominal CT of 12-year old girl with cystic abdominal mass.
Bilateral salpingo-oophorectomy was performed along with excision of tumor parts that were attached to peritoneal and omentectomy. During surgery, we found red-collored mixed with musin peritonel fluid, tumor on the right adnexa 13x17x21 cm attached to abdominal wall and omentum (Figure 16). Left adnexa tumor (Figure 17) was also found, sized 4x4x4 cm, with hair and sebasous tissue found within. Mature ovarian teratoma was revealed in pathological finding of both left and right ovaries with no healthy tissue in either ovaries.
Figure 16: Malignant ovarian tumor in right adnexa.
Figure 17: Cystic tumor of the left ovary.
Case 9
8-year old girl complained about abdominal mass since a year before hospital admission and vaginal discharge mixed with blood. In the abdominal palpation, there was solid mass size of 9x9x20 cm, fixated. Her abdominal CT revealed multilobulated septated with solid component in the middle and posterior side and calcification, size of 11.5x7.4x11.3 cm in pelvic cavity to abdominal cavity and suspected infiltration to the uterus and bowel, suggestive of malignancy. We performed tumor excision and right salpingo-oophorectomy after finding 18x13x7 cm right ovarian mass and its capsule was partly not intact on the superior side with omental walling-off and no intestinal infiltration, as seen in (Figure 18). The tumor was excised, weighed 950 gram and we found necrotic tissue with calcification within. Its pathological result showed grade 3 immature ovarian teratoma. Operative treatment was then continued with chemotherapy session (etoposid and cysplatin). Evaluation abdominal CT was performed five months later and showed no residual or recurrent mass.
Figure 18: Right ovarian teratoma found during operation.
Case 10
8-months-old female child was referred to our institution with suspicion of ovarian teratoma. The chief main complaint was abdominal enlargement since 2 weeks before she was brought to the hospital. The enlargement was in all abdominal quadrant and she had history of red cell transfusion due to anemia which was suspected to be caused by tumor hemorrhage. There was abdominal distention (Figure 19) with palpable mass in the lower right quadrant, sized 5x8 cm, fixated. Her abdominal ultrasound revealed heterogenic solid mass within abdominal cavity sized 6x4 cm, well-defined, regular edge with massive ascites. Her abdominal CT (Figure 20) showed enhancing mass with different fluid density component, calcification inside, sized 5.6x6.4x6.1 cm in pelvic cavity to right inguinal quadrant, ascites and spina bifida. Red, flat surfaced mass on the right adnexa (Figure 21), attached to the peritoneal and also mass on the left ovary (size of 3x2x2 cm), with soft surface and blueish which bleed easily were found intraoperatively. The right ovarian tumor was attached to the peritoneum, but no seeding to the surrounding tissue was found. We performed bilateral oophorectomy with uterus preservation and marsupialization of both left and right ovary and omentectomy. Pathological result revealed an ovarian dysgerminoma from both ovarian tumors. This patient was evaluated and prepared to receive chemotherapy.
Figure 19: Abdominal distention with palpable mass and ascites found in physical examination.
Figure 20: Abdominal CT showing mass in the pelvic cavity and right inguinal quadrant.
Figure 21: Operative finding; tumor size of 7x6.5x3 cm from the right adnexa.
Case 11
10-year-old girl was brought to the hospital, complaining of abdominal mass since 3 months prior to hospital admission. Her physical examination showed palpable abdominal mass, size of 7x7 cm, cystic and mobile in the medial and right lower quadrant. Abdominal CT (Figure 22) showed. Her AFP and beta-hCG levels were normal (1.1 ng/ml and < 2 mIU/ml). Right salpingo-oophorectomy was performed on this patient after discovering cystic mass sized 6.5x4.5x0.8 cm, weighing of 23.7 gram which can be seen in (Figure 23). Pathological finding of this tumor revealed mature cystic ovarian teratoma. She was then evaluated in the pediatric surgery clinic and her latest CT showed no residual nor recurrent mass.
Figure 22: Abdominal CT of 10-year-old girl with ovarian teratoma.
Figure 23: Right cystic ovarian mass in a 10-year-old girl.
Discussion
Ovarian teratomas are the most common germ cell tumor and the most common excised ovarian neoplasm. Teratomas develop from totipotent primordial cells. Teratomas are categorized as mature, immature, and malignant. Both mature and immature components are non-malignant tissues with uncertain metastatic potential. Malignant cells occur as other subtypes of germ cell tumors such as seminoma, choriocarcinoma, embryonal carcinoma and yolk sac tumor (YST). Mature teratoma is the most common (45%) tumor found in these serial cases (5 cases), while only 2 cases of immature teratoma, 2 cases of YST and 2 dysgerminoma in the last 4 years [3, 4]. Primary ovarian tumors are uncommon (only 2% of all cancer types in pediatric population). Annual incidence of both benign and malignant lesions in girls younger than 15 years was 1.6 cases per 100,000. Predominant pathology of ovarian tumors in children it was predominantly germ cell tumors which account for 77.4% of all ovarian tumors in all age group. Asian/ Pacific islanders had higher overall rates of germ cell tumors [4-6].
In our cases, most common clinical presentations are abdominal pain and palpable abdominal mass. The masses are usually mobile and palpable above the pelvic brim. Duration of symptoms is usually short with median duration of 2-4 weeks. Acute symptoms may present in case of ovarian torsion, rupture or hemorrhage, which could be found in less common symptoms such as abdominal distention (35%) and vaginal bleeding (10%). We only found one patient showing symptom of vaginal bleeding in grade 3 immature ovarian teratoma. Secondary symptoms include anorexia, nausea, vomiting and urinary frequency and urgency, which we did not find in our cases [4, 7]. Tumor markers that are commonly tested are alpha fetoprotein (AFP), beta-human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH). We usually evaluate serum AFP and beta-hCG levels in pediatric patients with abdominal mass. AFP increases in yolk sac tumor and teratocarcinoma and is usually re-measured for evaluating recurrence after treatment. Its elevation was shown in our immature ovarian teratoma and other malignancy cases. Beta-hCG elevation suggests presence of syncytiotrophoblas (seminoma, dysgerminoma, and choriocarcinoma), its rapid disappearance indicates complete tumor removal [4].
Imaging techniques that are commonly used are ultrasound and abdominal CT. Our cases with mature teratoma, showed cystic mass found both in abdominal ultrasound and/or CT. Three manifestations found in mature teratoma in ultrasound are cystic lesion, diffuse or partially echogenic mass and consisting of multiple thin and echogenic bands caused by hair in the cyst cavity, while mix of sold-cystic masses or nonspecific imaging are revealed in imaging in other germ cell tumors, like in immature ovarian teratoma or other ovarian malignancy. Immature teratomas are typically larger with perforation of its capsules, which are not always well defined [4, 5]. Staging system in ovarian germ cell tumors is based on its clinicopathological findings, which was arranged by Children’s Oncology Group (OCG) as seen in (Table 1) [8].
Table 1: COG Ovarian Germ Cell Tumors Staging System.
|
Stage I |
Limited to ovary (ovaries) peritoneal washings negative, tumor markers
normal after appropriate half-life decline |
|
Stage II |
Microscopic residual; peritoneal washings negative for malignant
cells, tumor markers positive or negative |
|
Stage III |
Lymph node involvement; gross residual or biopsy only, contiguous
visceral involvement (omentum, intestine, bladder); peritoneal washing positive
for malignant cells, tumor markers positive or negative |
|
Stage IV |
Distant metastases, including liver |
Management of germ cell tumors begins with surgical management. The goal of surgery is to completely evaluate the extent of disease, resect the tumor completely and spare uninvolved reproductive organs, especially in children. Conservative surgery with unilateral salpingo-oophorectomy and thorough contralateral ovarian inspection during surgery, with biopsy of suspicious lesion and careful staging is mandatory. Staging (by inspection, palpation and biopsy of any suspicious peritoneal or liver nodules) should be performed prior to surgical resection if characteristic between benign and malignant tumor is difficult to distinguish. Both ovarian inspection should be done intraoperatively, oophorectomy is performed if there’s no involvement of fallopian tube, while salpingo-oophorectomy is done when fallopian tubes are involved and because of the rich lymphovascular connections between tube and ovary. Unilateral salpingo-oophorectomy with preservation of normal uterus and contralateral ovary is an option in clinically early-stage disease. Evaluation of fertility in patients with unilateral oophorectomy showed that pregnancy rates appear to be the same as that of the general population. We mostly performed salpingo-oophorectomy of the involved ovary and fallopian tube to resect the tumor completely, in mature, immature and also malignant teratoma [4, 9-11].
As mentioned before, sparing uninvolved reproductive organ is one of management’s goal, and bilateral oophorectomy is rarely necessary, but there’s also another consideration in patient with malignancy that complete staging and resection should be the first goal of the surgery. In our cases of bilateral ovarian teratoma, we decided to perform bilateral salpingo-oophorectomy. In one case, one ovary side was pathologically malignant and the other one was benign, because it was rather difficult to decide its mass characteristic intra- and pre-operatively (since imaging studies revealing mixed consistency of mass, differentiation to benign and malignant tumors based on radiological finding is not possible) and complete staging and resection should be the first goal of surgery in malignancy, either to reduce the risk of recurrence and mortality in inadequate surgical removal, we did bilateral salpingo-oophorectomy. On another case of bilateral ovarian teratoma, both ovarian tumor was found to be malignant (dysgerminoma), we also performed bilateral salpingo-oophorectomy. Also, there is a consideration of ovarian preservation, which is size of the tumor (tumor size larger than 15 cm causes the ovary to be very thin owing to the large tumor, thus ovary preservation appeared to be difficult) [10, 12-14].
There are two options of surgical technique that can be performed, which are laparoscopy and laparotomy. Laparoscopy can be done in tumor with size of less than 75 mm, to take peritoneal fluid sampling, peritoneal inspection and tumor evaluation. If there is no seeding nor adhesion with easy tumor enucleation can be done, then laparoscopy shall be continued, otherwise if the tumor size is larger than 75 mm, with peritoneal seeding and adhesion, laparotomy must be performed. In these cases, we performed laparotomy surgery in all cases because the size of ovarian tumors was mostly larger than 77 mm, and the use of laparoscopy at our center is still limited [10]. Platinum-based therapy (regimen of cisplatin, etoposide and bleomycin) is the current regimens used in ovarian germ cell tumors. This regimen is given in malignant teratoma, including immature teratoma (especially in grade 2 and 3 of immature teratoma). Based on POG/CCG intergroup trial in 1990-1996, survival rate of ovarian cancer in chemotherapy of ovarian malignancy is shown in (Table 2), using the COG staging system. Chemotherapy regimens used at out center is cisplatin (P), etoposide (E) and bleomycin (B), where both cisplatin and etoposide are more commonly used. Therapy is discontinued after pathologic complete response and normal tumor markers [4, 8, 10]. Germ cell tumors are mentioned to be sensitive to radiotherapy, but cost of the cure may be too high if it compromises patient’s fertility, therefore radiation has been abandoned and multiagent chemotherapeutic program has been used for standard therapy in malignancy [10].
Table 2: Survival for ovarian cancer, POG/CCG intergroup trial 1990-96.
|
Stage |
Treatment |
6-year free survival (%) |
6-year survival (%) |
|
I |
S + PEB |
95 |
95.1 |
|
II |
S + PEB |
87.5 |
93.8 |
|
III |
S + HDP/EB vs PEB |
96.6 |
97.3 |
|
IV |
S + HDP/EB vs PEB |
86.7 |
93.3 |
B: bleomycin; E: etoposide;
B: bleomycin; P: cisplatin; HDP: high-dose cisplatin; S: surgery
Conclusion
Proper examinations (both physical and using diagnostic tools) should be done in diagnosing ovarian teratoma. Its management now seems to be adequate, which are surgery (tumor excision along with or without oophorectomy or salpingo-oophorectomy and chemotherapy (in malignant teratomas) are the standard therapy for ovarian teratoma and appeared to be quite satisfying result (10 out of 11 cases) in our experience with no signs of recurrence and good outcome.
Article Info
Article Type
Case SeriesPublication history
Received: Mon 22, Mar 2021Accepted: Wed 28, Apr 2021
Published: Mon 24, May 2021
Copyright
© 2023 Ageng Budiananti. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.OCR.2021.01.01
Author Info
Ageng Budiananti Fendy Matulatan
Corresponding Author
Ageng BudianantiResident of Pediatric Surgery, Dr. Soetomo General Academic Hospital, Airlangga University, Jawa Timur, Indonesia
Figures & Tables
Table 1: COG Ovarian Germ Cell Tumors Staging System.
|
Stage I |
Limited to ovary (ovaries) peritoneal washings negative, tumor markers
normal after appropriate half-life decline |
|
Stage II |
Microscopic residual; peritoneal washings negative for malignant
cells, tumor markers positive or negative |
|
Stage III |
Lymph node involvement; gross residual or biopsy only, contiguous
visceral involvement (omentum, intestine, bladder); peritoneal washing positive
for malignant cells, tumor markers positive or negative |
|
Stage IV |
Distant metastases, including liver |
Table 2: Survival for ovarian cancer, POG/CCG intergroup trial 1990-96.
|
Stage |
Treatment |
6-year free survival (%) |
6-year survival (%) |
|
I |
S + PEB |
95 |
95.1 |
|
II |
S + PEB |
87.5 |
93.8 |
|
III |
S + HDP/EB vs PEB |
96.6 |
97.3 |
|
IV |
S + HDP/EB vs PEB |
86.7 |
93.3 |
B: bleomycin; E: etoposide;
B: bleomycin; P: cisplatin; HDP: high-dose cisplatin; S: surgery





















References
1.
Oue T, Uehara S,
Sasaki T, Nose S, Saka R et al. (2015) Treatment and ovarian preservation in
children with ovarian tumors. J Pediatr Surg 50: 2116-2118. [Crossref]
2.
Smith HO, Berwick
M, Verschragen CF, Wiggins C, Lansing L et al. (2006) Incidence and Survival
Rates for Female Malignant Germ Cell Tumors. Obstetr Gynecol 107:
1075-1085. [Crossref]
3.
Terenziani M, D’Angelo P, Inserra A, Boldrini R, Bisogno
G et al. (2015) Mature and immature
teratoma: A report from the second Italian pediatric study. Pediatr Blood
Cancer 62: 1202-1208. [Crossref]
4.
Almen DV, Coran
AG, Adzick NS, Krummel TM, Laberge JM et al. (2012) Pediatric Surgery, 2-Volume
Set, 7th Edition Elsevier Saunders: Philadelphia 1: 529-547.
5.
Koulouris CR,
Penson RT (2009) Ovarian stromal and germ cell tumors. Semin Oncol 36:
126-136. [Crossref]
6.
Outwater EK,
Siegekman ES, Hunt JL (2001) Ovarian teratomas: tumor types and imaging
characteristics. Radio Graphics 21: 475-490. [Crossref]
7.
Pectasides D,
Pectasides E, Kassanos D (2008) Germ call tumors of the ovary. Cancer Treat
Rev 34: 427-441. [Crossref]
8.
Rescorla FJ
(2012) Pediatric germ cell tumors. Semin Pediatr Surg 21: 51-60. [Crossref]
9.
Grosfeld JL,
O’Neill JA, Fonkalsrud EW, Coran AG (2006) Pediatric Surgery. 6th ed. Mosby
Elsevier: Philadelphia 1: 593-614.
10. Vaysse C, Delsol M,
Carfagna L, Bouali O, Combelles S et al. (2010)
Ovarian germ cell tumors in children. Management, survival and ovarian
prognosis. A report of 75 cases. J Pediatr Surg 45: 1484-1490. [Crossref]
11. Gersgenson DM, Morris M, Cangir A, Kavanagh JJ, Stringer
CA (1990) Treatment of malignant germ cell tumors of the ovary with bleomycin,
etoposide, and cisplatin. 8: 715-720. [Crossref]
12. Ruttenstock EM, Saxena AK, Schwinger W, Sorantin E,
Hoellwarth ME (2010) Pediatric ovarian tumors--dilemmas in diagnosis and
treatment. Eur J Pediatr Surg 20: 116-120. [Crossref]
13. Cass DL, Hawkins E, Brandkt ML, Chintagumpala M, Bloss
RS et al. (2001) Surgery for ovarian masses in infants, children, and
adolescences in 102 consecutive patients treated in a 15-year period. J
Pediatr Surg 36: 693-699. [Crossref]
14. Sathyanarayama KV, Reddy
PS (2016) Teratomas in children: an institutional retrospective study. Asian
Pac J Health Sci 3: 18-23.
