Primary and Secondary Angiosarcoma of the Breast: Report of Two Cases and Review of the Literature
A B S T R A C T
Introduction: The breast's angiosarcoma is a rare entity, representing 1% of the breast's malignant neoplasms and is classified as primary and secondary; the latter is associated with radiotherapy and chronic lymphedema. Clinically both present as a voluminous and rapidly growing lesion. Surgery is the cornerstone of a treatment since it provides the most outstanding local control, whereas radiotherapy and chemotherapy have little impact on the disease. Similarly, the oncological prognosis is poor because this type of tumor has a high recurrence rate and overall, 5-year survival of only 20%.
Objective: Two cases of angiosarcoma of the breast (primary and secondary) are reported, both treated in the Breast Tumor Service and a bibliographic review of the disease is presented.
Materials and Methods: All breast cancer cases were reviewed in the Breast Tumor Service for two years.
Results: In the first clinical case, a 61-year-old patient was presented with a 5-month-old primary angiosarcoma in the right breast, treated with modified radical mastectomy. While in the second case, the clinical history of a 76-year-old woman with secondary breast angiosarcoma is related; the patient had a history of right breast cancer, treated ten years previously with conservative surgery, sentinel node and adjuvant radiotherapy. However, the woman presented local recurrence six years later, and she was operated on with a total mastectomy. Three years later, she developed an angiosarcoma in the surgical bed that warranted a wide resection of the chest wall.
Conclusion: The breast's angiosarcoma is an aggressive and rare tumor of unknown aetiology, since it has a high recurrence rate and poor survival. Its main prognostic factors are tumor volume and resection margins. Therefore, early diagnosis is essential because it allows complete resection of the lesion.
Keywords
Angiosarcoma, breast, primary, secondary, breast sarcoma
Introduction
Sarcomas of the breast include a rare heterogeneous group of neoplasms, accounting for less than 5% of soft tissue sarcomas and only 1% of malignant neoplasms in this region. In this sense, angiosarcoma is an even less frequent malignant neoplasm originating from the blood vessels' endothelial cells and is characterized by a rapid proliferation of neoplastic cells that extensively infiltrate local tissues [1, 2]. The first case of breast's angiosarcoma was reported in 1907 by Borrman, and the first one of primary origin was published in 1987 by Body [3]. In 1981, Maddox and Evans reported the first case in the thorax of a woman with a history of mastectomy for cancer [4]. In general terms, angiosarcoma is divided into primary and secondary. This clinical classification is of particular importance since it denotes several aspects of utility for the physician. First, there are differences between these two varieties concerning their age of presentation. Primary angiosarcoma is usually diagnosed between the ages of 30 and 50. In contrast, secondary angiosarcoma presents at a variable age; its peak incidence appears ten years after the end of radiotherapy treatments to the chest. Also, it is usually associated with chronic lymphedema (Stewart-Treves syndrome). Consequently, its age of presentation is variable [5, 6].
Second, regarding its histopathological characteristics, it can point out that primary angiosarcoma originates in the mammary gland's parenchyma. In contrast, the secondary type originates in the dermis and subcutaneous tissue [1, 2]. On the other hand, there are various histologies where each histological type presents different clinical characteristics, especially its evolution [3]. In summary, the breast's angiosarcomas are biologically more aggressive tumors than carcinomas originating in the mammary gland because they have a higher risk of local recurrence and lung relapse [7].
Risk Factors
The factors involved in the carcinogenesis of angiosarcoma can also be grouped according to their clinical classification. For example, in the primary tumor, patients have a history of exposure to carcinogenic agents, such as vinyl chloride, arsenic, and thorium dioxide. On the other hand, about the development of secondary angiosarcoma, the main associated factor is a chronic inflammation of the tissues: radiotherapy, radical mastectomy and axilla dissection [3, 8]. In other words, we can affirm that the frequency of the secondary tumor has increased in recent years due to the greater use of radiotherapy in breast-conserving surgery [4]. It is currently estimated that the risk of developing a soft tissue sarcoma in the breast region after the end of radiotherapy ranges from 0.01% to 0.02% per year. In comparison, for primary angiosarcoma, it is even higher, from 0.002 to 0.05% [9].
Clinical Findings
The most common clinical finding in primary angiosarcoma cases is the presence of a single blue tumor, usually large in volume that can sometimes occupy the entire breast due to its rapid growth. Multiple cutaneous and subcutaneous nodules are observed in the previously radiated site [3, 10]. These nodules generally appear six years after treatment is completed. However, they can appear as early as three years of follow-up or as late as twelve years of follow-up [4]. On the other hand, this neoplasm's biological behaviour is systemic, as usually happens in sarcomas; consequently, the axillary nodes' involvement is almost nil. However, it has a high incidence of distant disease. The most frequent metastasis sites are bone, lung, liver and the contralateral breast [10].
Histology
Angiosarcomas originate in the endothelium of blood vessels. Therefore, these tumors can develop in any part of the body. However, angiosarcoma of the breast represents up to 8% of them [11]. From a microscopic viewpoint, angiosarcomas present multiple vascular channels that anastomose with each other. All blood vessels show significant dilations since they are lined by giant endothelial cells. As in other neoplasms, angiosarcomas are classified according to their degree of differentiation. Thus, low-grade lesions show an infiltrative pattern but otherwise have an essential similarity concerning normal tissue, that is, the endothelial cells of the vascular channels present hyperchromatic nuclei with discrete atypia. While intermediate-grade lesions are similar, however, they present more excess of mitotic activity and endothelial tufts and multiple foci with a solid growth pattern [3].
In this sense, the pathologist must be able to make a differential diagnosis with other entities such as intramammary hemangioma, angiomatosis and pseudoangiomatous stromal hyperplasia. Finally, high-grade lesions represent a diagnostic challenge since they lose all similarity to endothelial tissue. For this reason, immunohistochemical markers are a fundamental tool. The main stains are CD31, CD34 and factor VIII; the best is CD31, which identifies endothelial membrane proteins individually [6, 8, 12]. On the other hand, a functional classification from the histopathological point of view is Rosen's, which groups angiosarcoma of the breast into three grades according to their level of differentiation: low, intermediate, and high [4]. Unlike other sarcomas, the histological grade does not correlate with the clinical course, particularly with the overall survival and the disease-free period, because the breast's angiosarcoma is considered an aggressive tumor. Regardless of its grade histological, this neoplasm has a high probability of developing metastasis in the short term [13]. In addition to the Rosen classification, three main histopathological angiosarcoma patterns have been described: type I is characterized by having vascular channels that invade breast tissue with little endothelial proliferation; type II presents papillary endothelial components. While type III has all of the above with areas of necrosis and hemorrhage [14].
Prognostic Factors
In general terms, up to 73% of patients with angiosarcoma will present some recurrence, which frequently occurs during the first year of follow-up [2]. The following factors have the most significant impact on the disease-free period: tumor volume, necrosis, number of mitoses, and histological grade [1]. Of all of them, the most important prognostic factor is tumor size since studies show that when a patient has a tumor > 5 cm, overall survival decreases from 91 to 50% [15]. In the same way, the size of the tumor favours the presence of close surgical margins ( < 5 mm), which in turn impacts the risk of local recurrence [5, 16]. Thus, angiosarcomas with a size > 10cm are those with the worst prognosis [9]. Overall survival of breast angiosarcoma is reported in 50% and recurrence-free survival in 3 and 5 years has been described in 20 and 0%, respectively [2].
In the same sense, the histological grade is also related to overall survival and is directly proportional; the more dedifferentiated the neoplasm is, the lower the survival rate. For example, well-differentiated angiosarcomas have a 5-year survival of 76%, while for poorly differentiated tumors, it is only 15% [8]. Finally, it can be pointed out that secondary angiosarcomas are more aggressive since primary angiosarcomas can even reach survival rates of up to 93 months. However, in general terms, global survival at five years is 73.3%, while for secondary angiosarcomas, it is only 3.5% [16]. In summary, a patient with a secondary angiosarcoma has a mortality rate twice as high [2, 5]. In the study by Ghareeb et al., the recurrence patterns are described according to the histopathological findings. Within these, necrosis and the number of mitoses> 10 high-power fields showed an increase in local recurrence. The above was added the clinical results of tumor size> 5cm with a morphological score> 15. It has an impact on the selection of patients who could benefit from adjuvant radiotherapy [1].
Treatment
There is no definitive consensus regarding the treatment of angiosarcoma of the breast since it is a rare neoplasm and clinical recommendations are based only on case reports or small series [7]. However, surgical resection with negative margins is the treatment of choice since it offers the possibility of cure, whereas radiotherapy and chemotherapy are reserved for selected cases [7]. Concerning systemic therapy, it is recommended for patients with unresectable local recurrence or with the presence of metastatic disease. The drugs most used are anthracyclines, taxanes, gemcitabine and ifosfamide. Response rates are variable depending on the drug, but in general, for the first line, it is 30% with a progression-free rate of 3.5 months, while for the following lines of treatment, it is less than 10% [17, 18]. However, there is no difference from the oncological point of view concerning the extent of surgery (radical or conservative), for example, recurrence for a mastectomy is 50%. In comparison, for a lumpectomy, it is 80% [6, 7].
On the other hand, adjuvant chemotherapy after complete resection of the lesion is a point of controversy. At the same time, some studies propose adjuvant radiotherapy in the case of conservative surgery. Research shows that the disease-free period increases to 57% at five years, when the patient receives adjuvant radiotherapy, being only 34% with surgery as the only treatment modality. However, there is no difference regarding overall survival [2]. Finally, the evidence shows that performing an axillary dissection does not improve the oncological prognosis and does increase postoperative morbidity [10].
Clinical Case 1
We present a 61-year-old female patient with no personal pathological history who began five months before treatment with autodetection of a nodule in the upper external quadrant -right breast-, which presented rapid and progressive growth. For this reason, she was referred to the Breast Tumors Service of our unit. During the initial physical examination, a tumor 15 cm in diameter was identified, replacing almost the entire breast. The neoplasm presented evidence of skin infiltration and ulceration of the areola-nipple complex, while the ipsilateral axillary region presented palpable adenopathy.
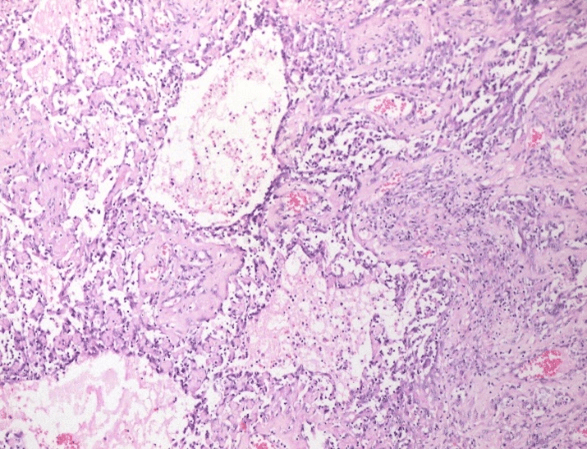
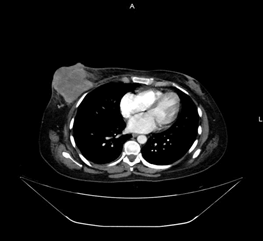
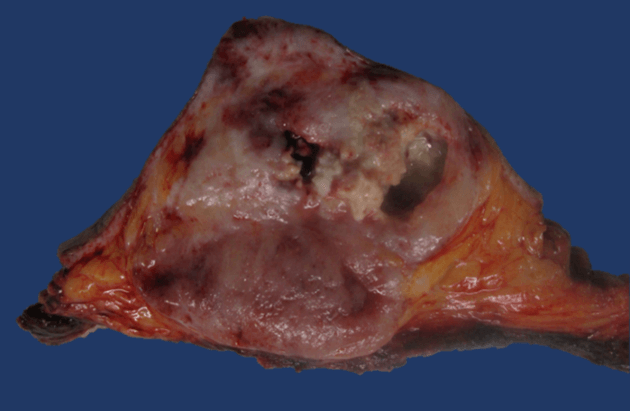
Therefore, a cutting needle biopsy was performed, which was reported compatible with a poorly differentiated malignant neoplasm with spindle-cell and epithelioid components (Figure 1). A thoracoabdominal contrast tomography was performed that ruled out infiltration to the chest wall or distant metastasis (Figure 2). Consequently, a modified radical mastectomy was performed. During the histopathological study, a fleshy-looking neoplasm of 11 cm in diameter and reddish in colour, but with greyish-white areas, were identified; When cutting the piece, a 3x2 cm cavitation with necrotic tissue was observed inside (Figure 3). Concerning the edges of the neoplasm, these were pushing and well defined. All margins of the surgical specimen were negative and axillary dissection revealed 27 lymph nodes with lymphoid hyperplasia. The final diagnosis was grade III angiosarcoma of the mammary gland, and due to the risk factors identified, adjuvant treatment was given with 60 Gy radiotherapy and to the rib cage. Subsequently, surveillance was started, and until now, she has a recurrence-free survival of 3 months.
Figure 1: Poorly differentiated malignant neoplasm with spindle-cell and epithelioid components.
Figure 2: Axial thoracic contrast tomography showing infiltration to the chest wall by right breast tumor.
Figure 3: Neoplasm of reddish and greyish-white areas. When cutting the piece, a 3x2 cm cavitation with necrotic tissue was observed inside.
Clinical Case 2
We present the case of a 76-year-old woman with a personal history of right breast cancer in 2010, who was treated with neoadjuvant chemotherapy, conservative surgery, and radical axillary dissection. Subsequently, she received adjuvant radiation therapy and hormone therapy (tamoxifen). The patient had a disease-free period of 6 years. During her follow-up consultation, a tumor was detected in the lower quadrant of the breast, the neoplasm presented a rapid and progressive growth, with clinical dimensions of 30 cm, for which a cutting needle biopsy was performed, the histopathological report was secondary angiosarcoma of the breast. A contrasted thoracoabdominal tomography was performed without identifying data of distant metastasis or involvement of the chest wall. Consequently, a total mastectomy was performed.
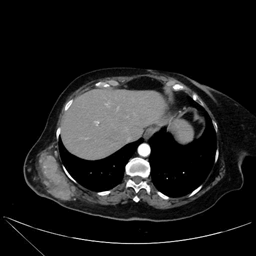
During the histopathological study, a 26 cm tumor was observed in its long axis, with data of necrosis and hemorrhage of 60% and extensive lymphovascular permeation, CD34 and CD31 positive, KI67 40%. The surgical margins were reported as tumor free. The patient did not receive any adjuvant treatment and began cancer follow-up. Subsequently, at 38 months of surveillance, the woman developed a nodule in the total mastectomy scar. Therefore, she had a cutting needle biopsy confirming recurrent angiosarcoma; in addition, extension studies were performed that documented the absence of infiltration into the chest wall without data of distant metastasis (Figure 4). Consequently, wide excision of skin and subcutaneous cellular tissue in the anterior thoracic wall and reconstruction with a facial flap was performed. She eventually restarted surveillance and has a disease-free period of 11 months and overall survival of 49 months.
Figure 4: Axial thoracic enhanced tomography showing tumoral activity after primary treatment. Resection with negative margins and flap reconstruction was needed.
Discussion
Angiosarcoma of the breast, a rare neoplasm present in <1% of malignant breast tumors, is a low-incidence entity. In the same way, its presentation is rare in our unit. In relation to the cases that we present, in the patient with primary breast angiosarcoma, it presented at 60 years of age, which contrasts with some cases in which they present on average at 40 years, however the series by Ming Yin et al. reports a mean age of 55 to 59 years, with a classic clinical presentation of a voluminous, fast-growing tumor, without lymph node involvement and treated with resection with free margins [2]. Regarding the pathological anatomy, a voluminous, high-grade tumor of 11 cm was documented, without lymph node involvement, which presents poor prognostic factors and based on the findings of Ghareeb et al., it was decided to grant adjuvant treatment with radiotherapy [1].
Secondary angiosarcoma presents an average of 6 years after previous radiotherapy treatment, which coincides with the presentation of our second case, a 71-year-old patient with breast angiosarcoma secondary to radiotherapy treatment, which was treated with surgery of rescue and a disease-free survival of 38 months, similar to the series by Taffurelli et al. that analysed the presentation of 24 cases of secondary angiosarcoma of the breast, presenting a new local recurrence in the chest wall, again requiring resection with free margins, achieving so far an overall survival of 49 months and disease-free survival after the last treatment of 11 months [19]. As shown in the cases presented and in relation to what is described in the literature, the treatment that has shown the best results in primary or secondary breast angiosarcoma despite its poor prognosis continues to be surgical resection with free margins, either in the first instance or rescue.
Conclusion
Breast angiosarcoma is a rare neoplasm that presents high rates of recurrence and mortality. Early diagnosis and tumor characteristics such as histological grade, tumor size, and resection with negative margins are the main prognostic factors and must be considered in decision-making, mainly for adjuvant treatment.
Article Info
Article Type
Case Reports and Review of the LiteraturePublication history
Received: Mon 15, Mar 2021Accepted: Tue 30, Mar 2021
Published: Wed 14, Apr 2021
Copyright
© 2023 Uriel Norberto Rivas-Mendoza. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RCO.2021.01.02
Author Info
Uriel Norberto Rivas-Mendoza Cristóbal Calixto-Martínez Raquel Valencia-Cedillo Jaime Resendiz-Colosia Jesús Alberto Sansón-Riofrio
Corresponding Author
Uriel Norberto Rivas-MendozaSurgical Oncology Resident, XXI Century National Medical Center, Mexico City, Mexico
Figures & Tables
References
1. Ghareeb ER, Bhargava R, Vargo JA, Florea AV, Beriwal S (2016) Primary and Radiation-induced Breast Angiosarcoma: Clinicopathologic Predictors of Outcomes and the Impact of Adjuvant Radiation Therapy. Am J Clin Oncol 39: 463-467. [Crossref]
2. Yin M, Wang W, Drabick JJ, Harold HA (2017) Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer 17: 295. [Crossref]
3. Wu WH, Ji QL, Li ZZ, Wang QN, Liu SY et al. (2019) Mammography and MRI manifestations of breast angiosarcoma. BMC Womens Health 19: 73.
4. Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F (2009) Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol 13: 147-150. [Crossref]
5. Masai K, Kinoshita T, Jimbo K, Asaga S, Hojo T (2016) Clinicopathological features of breast angiosarcoma. Breast Cancer 23: 718-723. [Crossref]
6. Molitoris JK, Chhabra A, Snider JW, Harvilla N, Okonkwo N et al. (2017) Intensification of Treatment for Angiosarcoma of the Breast with Accelerated Hyperfractionated Radiation, Hyperthermia, and Surgical Resection. Cureus 9: e1406. [Crossref]
7. Yin M, Mackley HB, Drabick JJ, Harvey HA (2016) Primary female breast sarcoma: clinicopathological features, treatment and prognosis. Sci Rep 6: 31497. [Crossref]
8. Mahdi Y, Rouas L, Malihy A, Lamalmi N, Alhamany Z (2018) Diagnostic difficulties of primary angiosarcoma of the breast: a case report. J Med Case Reports 12: 228.
9. Vorburger SA, Xing Y, Hunt KK, Lakin GE, Benjamin RS et al. (2005) Angiosarcoma of the breast. Cancer 104: 2682-2688.
10. Aljohani B, Al Twajeri T, Alameer A, Alzaydi T, Alawwad S et al. (2017) Clinicopathological features of breast angiosarcoma: A 16-years single-institution experience. Int J Surg Case Rep 37: 211-215. [Crossref]
11. Ragavan S, Lim HJ, Tan JWS, Hendrikson J, Chan JY et al. (2020) Axillary Lymph Node Dissection in Angiosarcomas of the Breast: An Asian Institutional Perspective. Sarcoma 2020: 4890803. [Crossref]
12. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ (2010) Angiosarcoma. Lancet Oncol 11: 983-991. [Crossref]
13. Guadagnolo BA (2009) Primary Angiosarcoma of the Breast: Clinicopathologic Analysis of 49 Cases, Suggesting That Grade is not Prognostic. Breast Dis Year 20: 404-405.
14. Kunkiel M, Maczkiewicz M, Jagiełło Gruszfeld A, Nowecki Z (2018) Primary angiosarcoma of the breast-series of 11 consecutive cases-a single-centre experience. Curr Oncol 25: e50- e53. [Crossref]
15. Adem C, Reynolds C, Ingle JN, Nascimento AG (2004) Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 91: 237-241. [Crossref]
16. Gutkin PM, Ganjoo KN, Lohman M, von Eyben R, Charville GW et al. (2020) Angiosarcoma of the Breast: Management and Outcomes. Am J Clin Oncol 43: 820-825. [Crossref]
17. D’Angelo SP, Munhoz RR, Kuk D, Landa J, Hartley EW et al. (2015) Outcomes of Systemic Therapy for Patients with Metastatic Angiosarcoma. Oncology 89: 205-214. [Crossref]
18. Dogan A, Kern P, Schultheis B, Häusler G, Rezniczek GA et al. (2018) Radiogenic angiosarcoma of the breast: case report and systematic review of the literature. BMC Cancer 18: 463. [Crossref]
19. Taffurelli M, Pellegrini A, Meattini I, Orzalesi L, Tinterri C et al. (2019) Secondary breast angiosarcoma: A multicentre retrospective survey by the national Italian association of Breast Surgeons (ANISC). Breast 45: 56-60. [Crossref]
