Pyrroloquinoline Quinone Enhances Cognition of Neuroinflammatory Alzheimer’s Disease Mouse Model via Mitochondrial Biogenesis Regulation
A B S T R A C T
Background: Mitochondrial biogenesis has been recently implicated to play an important role in Alzheimer’s disease (AD). Recently it has been reported that brains of AD patients show reduced expression in major genes and proteins such as PGC-1α involved in mitochondrial biogenesis. This led to the idea that enhancing mitochondrial biogenesis in AD, might represent a plausible strategy for AD treatment. Pyrroloquinoline quinone (PQQ) has been recently implicated in enhancing cognitive functions during aging; however, its effect on mitochondrial biogenesis in neuroinflammatory AD mouse model was not previously examined.
Objective: The aim of this project was to test the cognitive enhancement effect of PQQ in a neuroinflammatory mouse model mimicking AD, and whether PQQ is able to activate mitochondrial biogenesis in brains of our AD mouse model.
Methods: Neuroinflammatory AD mouse model was developed by Lipopolysaccharide (250 μg kg-1 body weight, i.p) injection for 7 days, followed by daily PQQ treatment (10 mg kg-1 body weight) on days 4-7. Cognitive functions were assessed using Y-Maze, Water-Maze and object recognition tests. Neurodegeneration was evaluated using H&E. Finally, mitochondrial proteins were measured using immunohistochemistry.
Results: PQQ treatment improved spatial recognition and working memory. PQQ treated mice brains showed decreased levels of neurodegeneration. Moreover, their brains showed greater amounts of both PGC-1α and the mitochondrial-membrane-bound protein cytochrome-c, indicating enhancement of mitochondrial biogenesis.
Conclusion: This study demonstrates the ability of PQQ to improve memory in neuroinflammatory AD model via enhancing mitochondrial biogenesis, which may represent an alternative mechanistic approach for treating AD.
Keywords
Alzheimer’s disease, mitochondrial biogenesis, pyrroloquinoline quinone, neuroinflammation, cognition, PGC-1α
Introduction
Mitochondrial biogenesis has been recently implicated to play a role in Alzheimer’s disease (AD). It is mainly regulated by the ‘Peroxisome Proliferator Activator Receptor Gamma-Coactivator 1α (PGC-1α)-Nuclear Respiratory Factor (NRF)-Mitochondrial Transcription Factor A (Tfam)’ pathway [1]. It was previously observed that brain areas affected by AD possess abnormalities in the mitochondrial distribution, in addition to aberrations in mitochondrial respiratory function, reactive oxygen species (ROS) production rates, and alterations in mitochondrial membrane potential [2]. In 2009, a study performed on hippocampal tissues of AD postmortem brains showed that mRNA expression of PGC-1α was significantly decreased when compared to age matched group. In 2012, Sheng B. and his colleagues demonstrated significant alterations in the expression levels of different proteins that are involved in the mitochondrial biogenesis pathway in hippocampal tissues from AD brains. Compared to age matched group, they found significantly reduced expression levels of NRF-1, NRF-2, Tfam and PGC-1α in AD hippocampal tissues, suggesting the impairment of mitochondrial biogenesis in AD brains [1]. This change was further confirmed in 2016, where the hippocampus of APP/PS1 mice showed a significant decrease in expression levels of PGC-1α [3]. In another study, treatment of APP23 mice with PGC-1α showed improvement in their cognitive function, along with significantly reduced deposition of Aβ [4]. Accordingly, based on the emerging evidence proving the involvement of mitochondrial biogenesis in the pathogenesis of AD, enhancing mitochondrial biogenesis might represent a novel treatment modality for targeting AD.
Pyrroloquinoline quinone (PQQ), also known as methoxatin, exists in different natural plants and food sources, for example, it is found in soybeans, spinach and field mustard [5, 6]. Several studies have tested the effect of PQQ in restoring and maintaining the normal cognitive abilities when administered to animal models. PQQ supplementation was found to prevent oxidative stress-mediated deterioration in memory, decrease cognitive damage induced by methylmercury toxicity [7-9], and reduce memory impairment stimulated by oxygen insufficiency [10]. In addition, when compared to Vitamin E, a 20 mg kg-1 dose of PQQ was found to have potency non-significantly different than 200 mg kg-1 Vitamin E in reversing age-related memory decline in rats [10]. In 2016, a clinical study was performed on 41 healthy elderly subjects testing the effect of PQQ on their cognitive functions. They were administered 20 mg of BioPQQ per day for 12 weeks, and their cognitive functions were evaluated using the Stroop and reverse Stroop test, and visual-spatial cognitive function. All results confirmed that PQQ prevents the brain function deterioration in elderly subjects, especially in attention and working memory [11].
Accordingly, a number of studies have tested the pro-cognitive properties of PQQ treatment either in ageing mouse models or human beings; however, few were oriented towards studying its underlying mechanism of action in subjects’ brains. In 2014, Zhao L. et al. was able to prove that PQQ salt inhibits the activity of glycogen synthase kinase-3, thus decreasing the amount of cerebral tau protein levels in APP/PS1 transgenic mice. In addition, they proved that their treatment also leads to increased activity of β-amyloid-binding alcohol dehydrogenase and reduced cerebral amyloid deposition [12]. Another study tested the effect of PQQ on cognition in 20 healthy human beings aged between 50 and 70-year-old. Upon examining the underlying reason behind its pro-cognitive effect, they found out that PQQ treatment leads to increased activity in the right prefrontal cortex (PFC) accompanied by an increase in regional cerebral blood flow (rCBF) and oxygen metabolism [13].
Nevertheless, to our knowledge, the relation between mitochondrial biogenesis enhancement and AD treatment with PQQ has never been addressed in previous studies. In 2010 however, Chowanadisai et al. tested the effect of PQQ on mitochondrial biogenesis in mouse hepatic cells, and results proved that PQQ is a positive regulator of this process by activation of PGC-1α via c-AMP response element-binding protein (CREB) phosphorylation [14]. In the same study, they found that PQQ causes an increase in the activity of mitochondrial enzymes; citrate synthase and cytochrome c oxidase, mitochondrial DNA content and cellular oxygen respiration. Moreover, there was an increase in nuclear respiratory factor activation (NRF-1 and NRF-2) and Tfam expression, which are downstream proteins in the PGC-1α pathway [14]. Since PQQ has been recently shown to improve cognitive functions in ageing mice and healthy elderly, this encouraged us to test its effect on cognition in a neuroinflammatory mouse model mimicking AD. Moreover, since mitochondrial biogenesis has been implicated to play a role in AD, and PQQ has been proven to enhance mitochondrial biogenesis in liver cells, we found it plausible to test whether its neuroprotective effects could be related to mitochondrial biogenesis.
Materials and Methods
I Animals and Housing
Adult male 4-6-month-old Swiss albino mice weighing 20-30 gm were used in the experiments. They were kept under constant conditions of temperature and humidity under a 12-hour light/dark cycles with continuous access to food and water. Cages were changed, cleaned and disinfected once weekly. All animal procedures were approved by the Ethics Committee of the German University in Cairo and were performed in accordance with the National Academies Guide for the Care and Use of Laboratory Animals (8th edition).
II Animals’ Treatment
A lipopolysaccharide (LPS) model was developed based on the work of Lee et al. in 2008, which studied the cognitive impairment as well as the AD pathological hallmarks of this neuroinflammatory in-vivo mouse model. In this study, they proved that repeated intraperitoneal injection of LPS (strain 055:B5) with a dose of 250 μg kg-1 body weight induces memory impairment. Moreover, they proved that this treatment regimen resulted in increased beta- and gamma-secretase activities accompanied with the increased expression of amyloid precursor protein (APP) leading to the accumulation of Aβ1-42 in the hippocampus and cerebral cortex of mice brains [15]. This model was further confirmed in our research group where we were able to show accumulation of Aβ1-42 protein in the cerebral cortex of the mice and decline in memory and cognition [16, 17].
In our study, mice were randomly divided into four different treatment groups; a control group, an LPS group, a PQQ treated group and finally a PQQ control group. Mice injection plan lasted for seven consecutive days, in which they were daily injected LPS (Sigma Aldrich, Strain: Escherichia coli, 055:B5. Dose: of 250 µg kg-1 body weight) or its vehicle. Followed by; a second treatment injection of PQQ (Mitsubishi gas company, Dose: 10 mg kg-1 body weight) or its vehicle starting day number four. The chosen dose of PQQ was based upon previous in-vivo studies targeting brain injuries in different mouse models [18, 19]. The time interval between LPS and PQQ injections was two hours, and all injections were administered intraperitoneally.
III Behavioural Testing
i Novel Object Recognition Test
The novel object recognition task is a method used to measure a specific form of recognition memory based on the spontaneous behaviour of animals. This procedure has been performed according to the work of Bevins & Besheer in 2006 [20]. The apparatus used for this test was a white wooden painted box (40×40×60 cm). Two distinctive objects were used in the training and test sessions; a blue wooden cube was used as the sample object and a yellow wooden pyramid was used as the novel object.
In the training session, sample objects were put in the back left and right corners of the apparatus. Mice were placed at the mid-point of the wall opposite to the objects. Each mouse was allowed to explore the objects (sample-object exposure) for ten minutes, and then it was returned to its colony. The training to testing interval was set to be two hours. In the testing session, one of the sample objects was replaced with the novel object. Each animal was placed in the same position in the apparatus as the training session. The test session interval was set to be five minutes, which was video recorded using a semi-professional camera fixed above the apparatus, for archiving and for results analysis.
Object related behaviour was scored using “Direct Contact Scoring” method. Any contact of the mouse with the objects using its mouth, nose or paw intending to explore it, was considered ‘direct contact’. Results of the novel-object recognition test are presented by Discrimination Ratio (D.R), which is the time (in seconds) spent by the animal exploring the novel object divided by the time spent exploring both objects.
ii Y Maze Test
This test is classified as “spatial learning task” based on the working memory of the rodents [21]. The test depends on the memory of the mouse remembering which arm of the maze was recently visited to avoid it and go to another one. The procedure of the test was done after the work of Arai et al. [22].
The apparatus used for this test is a y-shaped apparatus consisting of 3 arms (32 cm length and 10 cm height) labelled ‘A, B and C’ with 120° angle between each arm. In the testing session, mice were placed in the center of the maze and were left to explore it with all the arms open for eight minutes. Results were manually recorded during the test by writing the sequence of arms that the mouse enters (e.g., ABCACBA). For an entry to be counted, the mouse must have its four paws and its tail inside the arm. Every three consecutive arms entries (e.g., AAB, BCA, BCB...) represented one “alternation”, and each three consecutive arms choices of three different arms were counted as a correct choice of spontaneous alternation. Mice which entered nine arms only or less per test session were excluded from the study. Results were then expressed as “Spontaneous alternation percentage”, where the number of alternations is divided by the total number of possible alternations, and the result is multiplied by 100.
iii Morris Water Maze
Place or spatial learning paradigm on Morris Water Maze (MWM) was developed in 1984 by Richard G. Morris [22]. The theory behind this test is that the animal learns to use distal cues to navigate a direct route to the hidden platform, even if it is placed in different random positions in the water tank. This experiment was done after the work of Nunez J. et al., where animals are placed in a water tank filled with opaque coloured water, where they are supposed to reach a hidden escape platform. They mainly rely on external maze cues to find their escape route as the platform is hidden. Hence, they cannot rely on their visual or hearing abilities. The task becomes much easier to the animals as they become more familiar with the tank and its surrounding environment [23, 24].
For the maze training, an escape platform (15×15cm) was placed in the ‘North-East’ quadrant of a round stainless-steel pool (diameter: 150 cm, depth: 60 cm) with one inch exposed above the water surface to be visible for the mice. Each mouse was subjected to three consecutive trials by placing it first on the platform for twenty seconds, then putting it on one of the starting positions (e.g., North) and allowing it to search for the platform for a maximum of 60 seconds. In the experimental trial, the tank was filled with water one inch above the platform to cover it completely. Each mouse was subjected to 60 seconds 12 trials, 3 trials for each start position, where the mouse was put in the tank facing the wall and was allowed to search for the hidden platform for 60 seconds.
All the experimental trials were video recorded using a semi-professional camera fixed above the apparatus, for archiving and for results calculation. In the test sessions, time for each mouse to reach the platform was recorded as well as the distance it covered has been recorded by analysing the archived videos using Kinovea programme. Results were assessed by comparing the mean time (in seconds) and distance (in centimetre) for each animal group.
IV Haematoxylin and Eosin Staining
Mice were sacrificed by cervical dislocation and their brains were collected. Mice’s brains (n=3) were fixed in 10% formalin saline for twenty-four hours. After washing the samples with water, a serial dilution of alcohol (methyl, ethyl and absolute ethyl) was used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56°C hot air oven for 24 hours. Paraffin bees wax tissue blocks were prepared for sectioning using a sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by haematoxylin and eosin stain for routine examination using a light microscope.
V Immunohistochemistry
Serial sections of 5 μm thicknesses were cut from the paraffin blocks of the brain samples (n=3) and applied onto glass slides coated with poly-lysine. Deparaffinization of the tissue sections was then done using xylene, then rehydration was done using a serial dilution of ethanol. Tissue sections were boiled in 10 mM citrate buffer, pH (6.0), then left to cool at room temperature. Ultra V block was applied, and sections were incubated in a moist chamber at room temperature. Primary antibodies of either cytochrome c (Santa Cruz Biotechnology INC., USA) or PGC-1α (Millipore Corp., USA) in 10% bovine serum albumin were then applied to the sections. Sections were then treated with biotinylated secondary goat antibodies (Invitrogen, Germany). After incubation with streptavidin peroxidase (Carl Roth, Germany), visualization of the antibodies was done by adding the peroxidase-compatible chromogen DAB mixture. Slides were washed counterstained with Mayer-Haematoxylin (VWR, Germany). Finally, sections were rinsed, dehydrated in graded series of alcohol, cleared in xylene and coverslipped with histomount mounting medium.
VI Statistical Analysis
Statistical analysis of the groups was presented as means ± standard error of the mean (S.E.M) using GraphPad Prism software (version 6). Data analysis was done using the one-way analysis of variance (ANOVA) procedure followed by Tukey’s multiple range test. Upon analysis, P<0.05 was considered a significant value. In all of the experimental procedures, both the experiment operator and the data analyst were blinded.
VII Chemicals Supplier
Lipopolysaccharide, Strain: Escherichia coli, 055:B5 was purchased from Sigma Aldrich, Germany. Pyrroloquinoline Quinone was kindly provided as a gift from Mitsubishi gas company, Japan. Cytochrome c antibody was purchased from Santa Cruz Biotechnology INC., USA and PGC-1α antibody from Millipore Corp., USA. Biotinylated secondary goat antibodies were purchased from Invitrogen, Germany, streptavidin peroxidase from Carl Roth, Germany and finally Mayer-Haematoxylin was purchased from VWR, Germany.
Results
I Effect of PQQ on Behavioural Performance and Cognitive Function in Neuroinflammatory Mouse Model
The first step of our work was to assess the effect of PQQ on behavioural performance and cognitive function of the LPS neuroinflammatory mouse model. First, the object recognition test was performed to measure the non-spatial recognition memory of the mice based on their spontaneous behaviour. The Y-maze test was performed to measure the spatial learning memory of the mice based on their short-term working memory. Finally, the Morris water maze test was performed to measure the spatial memory of the mice based on their working memory, reference memory and task strategy.
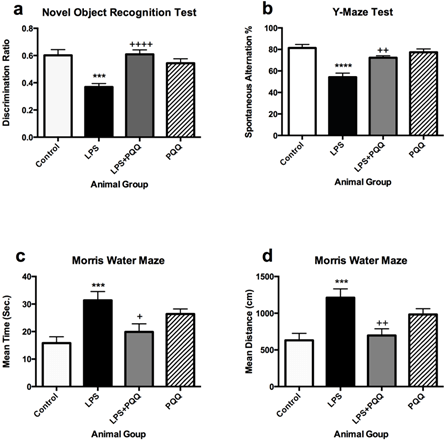
Mice which were administered LPS only showed significantly lower discrimination ratio when compared to the control group in the object recognition test (Figure 1a). They also showed a significant decrease in the spontaneous alternation % in the Y-maze test (Figure 1b), and they spent significantly more time swimming and covered longer distance to reach the hidden platform in the Morris Water Maze test when compared to the control group (Figures 1c & 1d) although both groups underwent the same training sessions.
Figure 1: PQQ enhances cognitive functions of the neuroinflammatory mouse model: a) PQQ enhanced the behavioural recognition memory of the LPS mouse model by increasing the discrimination ratio (n=10). b) PQQ enhanced the short-term working memory of LPS-mouse model by increasing the spontaneous alternation % (n=7). PQQ enhanced the working and reference memory of LPS- mouse model by c) time spent and d) decreasing distance covered searching for the escape platform (n=10).
Data represent mean ± S.E.M. ***p≤0.001 and ****p≤0.0001 relative to control group. +p≤0.05, ++p≤0.01 and ++++p≤0.0001 relative to LPS group.
On the other hand, the animal group treated with PQQ showed higher discrimination ratio when compared to the LPS group in the object recognition test (Figure 1a), indicating that they could retain a higher preference for the novel object over the old object, almost reaching same levels of the control group. Also, the action of LPS was fortunately reversed in the Y-maze test, in which mice treated with PQQ showed a significant increase in the alternation % when compared to the LPS group reaching close levels to the control (Figure 1b). The same pattern was observed in the Morris Water Maze test in which PQQ treated mice spent a comparable time to the control group and significantly less time when compared to the LPS group (Figure 1c). Corresponding results were observed in the distance covered by the mice to reach the platform, in which the same animal group also showed significantly shorter distance than the LPS group (Figure 1d). The animal group which was administered PQQ only did not show any significantly different results compared to the control group in the 3 tests performed (Figure 1). These results indicate that PQQ is able to improve non-spatial recognition memory, short-term working memory, reference memory as well as task strategy based on the three performed behavioural tests.
II Effect of PQQ on Neurodegeneration in Neuroinflammatory Mouse Model
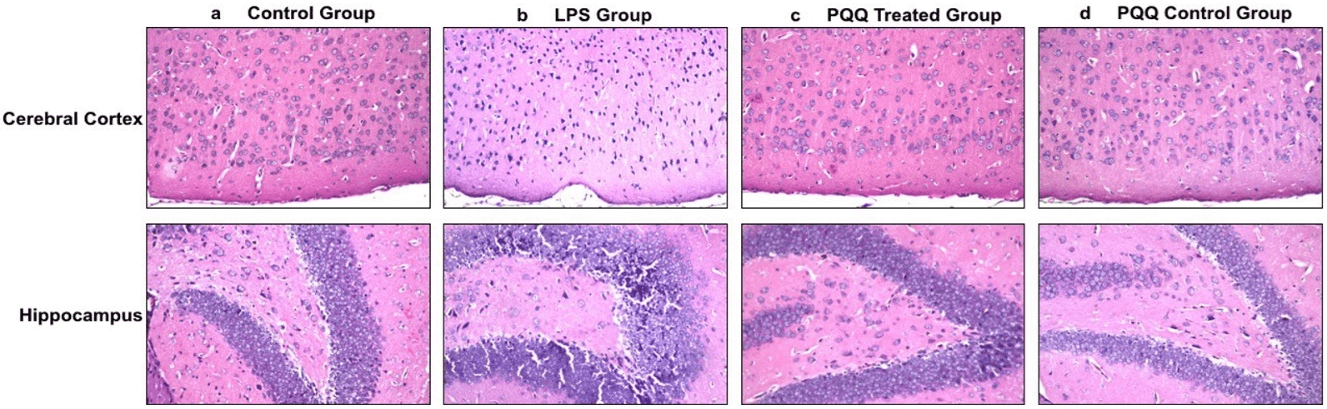
After confirming that PQQ is able to enhance behaviour and cognition in the neuroinflammatory AD mouse model, the next step was to observe its histological effect on the mice’s brains, and whether it could reverse the LPS neurodegenerative effect. This was done by H&E staining of the mice’s brains. In the control group, different brain areas including the meninges, cerebral cortex, hippocampus, and striatum showed normal histological structure (Figure 2a). On the contrary, the LPS induced group showed nuclear pyknosis in the cerebral cortex as well as in the hippocampus at the subiculum and fascia dentate (Figure 2b). Fortunately, the neuroinflammatory mouse model treated with PQQ showed no histological alterations in the cerebral cortex, hippocampus, and striatum (Figure 2c). Figures of the PQQ treated group show that PQQ was able to prevent the neurodegenerative effect of LPS and return neurons to their normal state as in the control group. Finally, the PQQ control group showed no histopathological alterations, as in the control group (Figure 2d).
Figure 2: PQQ prevents neurodegeneration in brains of neuroinflammatory mouse model: Histopathological structure of cerebral cortex (Magnification: 40x) and hippocampal (Magnification: 40x) regions of brains of the four animal groups; a) Control group, b) LPS group, c) PQQ treated group and d) PQQ control group.
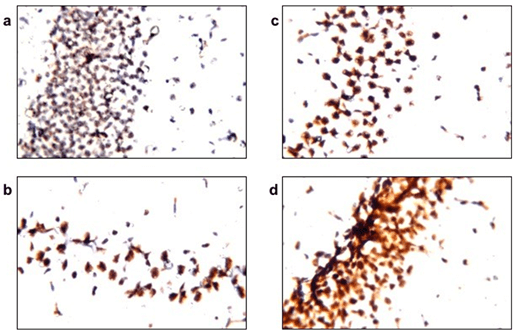
Figure 3: PQQ enhances mitochondrial biogenesis process in neuroinflammatory mouse model: Immunohistochemical staining with PGC-1α mAB of the four animal groups’ brains, hippocampus; a) Control group, b) LPS group, c) PQQ treated group and d) PQQ control group. Magnification: 80x.
III Effect of PQQ on Mitochondrial Biogenesis
To assess the effect of PQQ on mitochondrial biogenesis, brain samples obtained from the treated mice were immunostained with a marker for mitochondrial biogenesis; PGC-1α. Subsequently, brains were immunostained with cytochrome c, a mitochondrial membrane-bound protein, for estimation of the mitochondrial count in the mice’s brains. Results revealed that the LPS group (Figure 3b) showed decreased staining intensity of PGC-1α when compared to the control group (Figure 3a). However, PQQ treated group (Figure 3c) showed a tendency of increased PGC-1α immunostaining when compared to the LPS group.
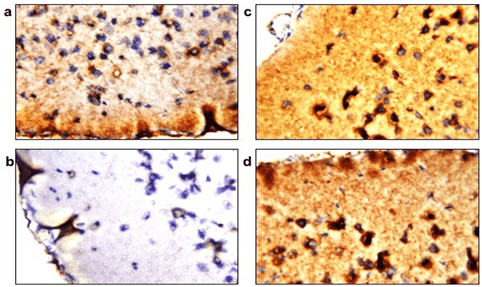
Estimated mitochondrial count showed a significant decrease in the LPS group (Figure 4b) when compared to the control group (Figure 4a) as shown by the decreased immunoreactivity of cytochrome c protein in the brain samples. However, immunoreactivity was retained in brains of the PQQ treated group (Figure 4c) in amounts comparable to the control group. Finally, the PQQ control group (Figure 4d) showed similar immunoreactivity of cytochrome c as the control.
Figure 4: PQQ increases cytochrome c in neuroinflammatory mouse model: Immunohistochemical staining with Cytochrome c mAB of the four animal groups’ brains; a) Control group, b) LPS group, c) PQQ treated group and d) PQQ control group. Magnification: 80x.
Discussion
In our study, we aimed to assess the effect of a natural compound, Pyrroloquinoline Quinone (PQQ) on a neuroinflammatory neurodegenerative in-vivo mouse model mimicking AD. The idea of the study was anticipated after the observation that PQQ could improve memory and cognitive abilities in ageing mice. In addition, it was previously proven to affect mitochondrial biogenesis, which is an important pathological pathway involved in AD. However, this observation has been proven in hepatic cells and to our knowledge was not examined in AD models.
The first aim of our study was to examine the effect of PQQ treatment on a neuroinflammatory mouse model mimicking AD to perceive its effect on the behavioural changes of the animals regarding memory and learning. Results of the behavioural tests revealed that LPS causes memory deterioration and cognitive impairments, as reported by several previous studies [15, 21, 25-27].
On the other hand, all results of the three performed tests showed that PQQ reversed the cognitive impairment caused by LPS. Several recent studies support those findings, proving that PQQ has the ability to restore cognitive impairments and maintain them at normal levels in brain injury model and oxidative stress animal model [7, 28]. Cognitive enhancement effect of PQQ was reported in studies performed on ageing mice. PQQ when compared to Vitamin E in reversing age-related memory decline in rats, was found to have the same potency [10]. The effect of PQQ disodium salt (BioPQQ) on cognitive functions was also seen in a study carried out in 41 ageing healthy humans, where PQQ prevented the brain functions deterioration, especially in attention and working memory [11]. In 2017, another study also proved that a nutraceutical formula containing PQQ enhances cognitive functions in a transgenic Alzheimer’s disease mouse model (5XFAD) [29]. To our knowledge, this is the first study to confirm cognitive enhancement effects of PQQ in a neuroinflammatory mouse model mimicking AD.
After confirming the pro-cognitive effect of PQQ in our AD mouse model, the following step was to test if it prevents the neurodegenerative effect induced by LPS in the mice’s brain. Analysis of the mice’s brains was performed using H&E staining. LPS was able to cause severe neurodegeneration in different brain areas including the brain cortex, striatum, and hippocampus, which is in accordance with previously reported results [30-34]. PQQ treatment which started four days after LPS injection was able to prevent this action leading to restoration of damaged neurons.
The neuroprotective effect of PQQ has been previously discussed in several in-vitro models. Only few in-vivo studies tested the effect of PQQ’s neuroprotective effect. This was proven in a rat model of reversible middle cerebral artery occlusion (rMCAo), a rat model of glutamate-induced toxicity, also, it was found to exert a protective effect in a Parkinson’s Disease model induced by medial forebrain bundle injection with rotenone [18, 35-37]. Nevertheless, our study is the first to study the effect of PQQ on neurodegeneration in a neuroinflammatory mouse model mimicking AD pathology. After confirming the neuroprotective effect of PQQ, in addition to its ability in restoring memory deterioration and cognitive impairment in the AD neuroinflammatory animal model, our target was to know its mechanism of action and whether mitochondrial biogenesis is involved in its neuroprotective and cognitive improvement effects. Accordingly, one of the main proteins involved in the signaling pathway controlling mitochondrial biogenesis (PGC-1α) was quantified.
Results of this test revealed a decrease in the PGC-1α protein in the LPS group when compared to the control group. The effect of LPS on mitochondrial biogenesis has been controversial. From previous studies, it seems that acute LPS treatment leads to increased mitochondrial biogenesis as a compensatory mechanism to the mitochondrial dysfunction that it causes [38-40]. While chronic induction of neuroinflammation with LPS might lead to down-regulation in the key players of mitochondrial biogenesis, which is in accordance with our preliminary results [37, 41-49]. A possible explanation for this controversy could be that mitochondrial biogenesis increases at the beginning as a repair mechanism. However, after a long period of LPS injection, this compensatory mechanism is expected to fail, leading to a decrease in mitochondrial biogenesis. Interestingly, PQQ treatment increased the levels of PGC-1α in our AD model. In addition, there was no difference between the control group and the PQQ treated group, which confirms that PQQ treatment shows an increase in the PGC-1α content after LPS administration to a level close to the control group.
We suggest that PQQ could retain a normal level of mitochondrial biogenesis in brain tissue after inducing neuroinflammation via LPS. PQQ has been known for its capability to increase mitochondrial content in cells, proven by in-vivo experiments depriving animals from PQQ supplementation, where mitochondrial DNA content was found to be reduced in liver cells [50, 51]. Nevertheless, it wasn’t until 2009 when Chowanadisai and his colleagues confirmed its positive effect on mitochondrial biogenesis, specifically on the PGC-1α signaling pathway via cAMP response element-binding protein (CREB) phosphorylation and activation in mice hepatic cells [14]. To our knowledge, the effect of PQQ on PGC-1α pathway responsible for enhancing mitochondrial biogenesis in AD brains not tested before. However, previous results performed on muscle and liver tissues are in accordance with our findings. Kuo et al. investigated the effect of PQQ on the PGC-1α pathway in denervation induced muscle atrophy mouse model [52]. Afterwards, Singh et al. discussed the protective effect of PQQ as well as its mitochondrial biogenesis boosting effect in hepatic tissue against rotenone induced mitochondrial oxidative stress in rats in addition to mitochondrial dysfunction in naturally ageing rat model [53]. The results of both studies prove that PQQ is a positive regulator of mitochondrial biogenesis signaling pathway. Interestingly, recently PQQ was reported to increase mitochondrial biognesis in a rotenone-induced parkinson's model, They reported an increase in the expression of PGC-1α and downstream genes in the mitochondrial biogenesis pathway [37, 54].
Also, in accordance with our findings Adami et al. studied the pre-synaptic mitochondrial function in hemizygous (+/-)TgMcGill-R-Thy1-APP rats, which showed a decrease in respiratory control ratio and spare respiratory capacity, coupled with deficits in complex I enzymatic activity. This transgenic AD model when supplemented with PQQ along with their complete diet showed reversal of their brain bioenergetics deficits along with improvement in the cognitive impairments [55]. Moreover, Darell et al. proved that treating AD transgenic mouse model with a nutraceutical formulation containing PQQ affected positively mitochondrial function in the mice brains as demonstrated by the decrease in ROS levels and membrane hyperpolarization, along with the elevated ATP levels and respiratory status [29].
These previous findings in AD models indicate improvement in mitochondrial functions and bioenergetics, and our study indicates that these improvements could be related to increase in the mitochondrial biogenesis by activating the PGC-1α pathway. To confirm the increase in mitochondrial proteins after PQQ treatment, a mitochondrial protein was chosen as a mitochondrial marker. The cytochrome complex is a mitochondrial inner-membrane hemeprotein that belongs to the cytochrome c family. Accordingly, its immunohistochemical staining in brains of our different mice groups was performed.
Results revealed that the staining intensity of cytochrome c was decreased in the LPS-injected group, which reflects low mitochondrial number upon treatment with LPS. In the current study, cytochrome c protein staining showed a reduction in the brains of LPS treated group. The brains of the neuroinflammatory mice group treated with PQQ immunostaining showed that cytochrome c was retained after the reduction caused by LPS injection. Interestingly, our study is the first to report that PQQ treatment in a neuroinflammatory neurodegenerative mouse model causes an increase in the total content of an important mitochondrial protein – cytochrome c, which might suggest a boosting action on the mitochondrial number in brain cells. Those results are in accordance with several studies demonstrating that PQQ causes an increase in the total mitochondrial number. Those results were observed in in-vitro studies performed on brain, heart and liver cell cultures, as well as in-vivo studies performed on liver and heart tissues [14, 53, 56-58]. More studies are required to confirm the increase in mitochondrial biogenesis in AD brains after treatment with PQQ.
Conclusion
To conclude, our study was able to prove that PQQ has positive effects on cognition and memory of a neuroinflammatory AD mouse model. The mechanism of action of PQQ seems to be related to an increase in mitochondrial biogenesis in brain tissue. Further future studies are suggested to measure content of further proteins involved in this signaling pathway, along with their expression levels. It is also suggested to measure mitochondrial marker proteins and indicate the mitochondrial content in brain tissue to interpret the overall effect of PQQ on mitochondrial count.
Author Contributions
Both authors have contributed to this work equally regarding; design of the work, interpretation of data, drafting and revising the work content, approval of the final version to be published, and in agreement to be accountable for all aspects of the work.
Acknowledgement
We would like to thank Mitsubishi Gas Company for providing us with the compound Pyrroloquinoline Quinone (PQQ). In addition, we acknowledge Professor Dr. Adel Bakeer for his help in the analysis of the H&E and immunohistochemistry work. We would also like to thank Mr. Ahmed M. Abdelgawad, Mr. Ibrahim M. Ibrahim and Mr. El-Sayed A. El-Sayed, the pharmacology lab technicians for their great help.
Conflicts of Interest
Pyrroloquinoline Quinone was kindly provided as a gift from Mitsubishi Gas Company.
Ethical Approval
No humans were used in this study. Legal and ethical approvals were obtained from the (GUC Ethics Committee) prior to initiation of the research work carried out on animals. The investigation conforms to the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (8th edition).
Abbreviation
AD: Alzheimer’s Disease
Aβ: Amyloid Beta
APP: Amyloid Precursor Protein
APP/PS1: Amyloid Precursor Protein/Presenilin 1
ANOVA: Analysis of Variance
CREB: c-AMP Response Element-Binding Protein
DR: Discrimination Ratio
H&E: Heamatoxylin and Eosin
LPS: Lipopolysaccharide
Tfam: Mitochondrial Transcription Factor A
MWM: Morris Water Maze
NS/PCs: Neuronal Stem and Progenitor Cells
NRF: Nuclear Respiratory Factor
PGC-1α: Peroxisome Proliferator Activator Receptor Gamma-Coactivator 1α
PQQ: Pyrroloquinoline Quinone
ROS: Reactive Oxygen Species
rCBF: Regional Cerebral Blood Flow
rMCAo: Reversible Middle Cerebral Artery Occlusion
PFC: right Prefrontal Cortex
S.E.M: Standard Error of Mean
Article Info
Article Type
Research ArticlePublication history
Received: Tue 02, Nov 2021Accepted: Wed 17, Nov 2021
Published: Thu 02, Dec 2021
Copyright
© 2023 Reham M. Abdel-Kader. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.04.01
Author Info
Engy A Fadel Reham M. Abdel-Kader
Corresponding Author
Reham M. Abdel-KaderDepartment of Pharmacology, Toxicology and Clinical Pharmacy Faculty of Pharmacy and Biotechnology, German University in Cairo (GUC), Egypt
Figures & Tables
Data represent mean ± S.E.M. ***p≤0.001 and ****p≤0.0001 relative to control group. +p≤0.05, ++p≤0.01 and ++++p≤0.0001 relative to LPS group.
References
1. Sheng B, Wang X, Su
B, Lee HG, Casadesus G et al. (2012) Impaired mitochondrial biogenesis
contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem
120: 419-429. [Crossref]
2. Dragicevic N,
Mamcarz M, Zhu Y, Buzzeo R, Tan J et al. (2010) Mitochondrial amyloid-beta
levels are associated with the extent of mitochondrial dysfunction in different
brain regions and the degree of cognitive impairment in Alzheimer's transgenic
mice. J Alzheimers Dis 20: S535-S550. [Crossref]
3. Ettcheto M, Petrov D, Pedros I, Alva N, Carbonell T et al. (2016) Evaluation
of Neuropathological Effects of a High-Fat Diet in a Presymptomatic Alzheimer's
Disease Stage in APP/PS1 Mice. J Alzheimers Dis 54: 233-251. [Crossref]
4. Katsouri L, Lim YM, Blondrath K, Eleftheriadou I, Lombardero L et al. (2016)
PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β
generation by reducing β-secretase in an Alzheimer's disease model. Proc
Natl Acad Sci U S A 113: 12292-12297. [Crossref]
5. Noji N, Nakamura T,
Kitahata N, Taguchi K, Kudo T et al. (2007) Simple and sensitive method for
pyrroloquinoline quinone (PQQ) analysis in various foods using liquid
chromatography/electrospray-ionization tandem mass spectrometry. J Agric
Food Chem 55: 7258-7263. [Crossref]
6. Kumazawa T, Sato K,
Seno H, Ishii A, Suzuki O (1995) Levels of pyrroloquinoline quinone in various
foods. Biochem J 307: 331-333. [Crossref]
7. Ohwada K, Takeda H,
Yamazaki M, Isogai H, Nakano M et al. (2008) Pyrroloquinoline Quinone (PQQ)
Prevents Cognitive Deficit Caused by Oxidative Stress in Rats. J Clin
Biochem Nutr 42: 29-34. [Crossref]
8. Zhang P, Xu Y, Sun
J, Li X, Wang L (2009) Protection of pyrroloquinoline quinone against
methylmercury-induced neurotoxicity via reducing oxidative stress. Free
Radic Res 43: 224-233. [Crossref]
9. Zhang P, Xu Y, Li
L, Jiang Q, Wang M (2009) In vitro protective effects of pyrroloquinoline
quinone on methylmercury-induced neurotoxicity. Environ Toxicol Pharmacol
27: 103-110. [Crossref]
10. Takatsu H, Owada K,
Abe K, Nakano M, Urano S (2009) Effect of vitamin E on learning and memory
deficit in aged rats. J Nutr Sci Vitaminol (Tokyo) 55: 389-393. [Crossref]
11. Itoh Y, Hine K,
Miura H, Uetake T, Nakano M et al. (2016) Effect of the Antioxidant Supplement
Pyrroloquinoline Quinone Disodium Salt (BioPQQ™) on Cognitive Functions. Adv
Exp Med Biol 876: 319-325. [Crossref]
12. Zhao L, Gong N, Liu
M, Pan X, Sang S et al. (2014) Beneficial synergistic effects of microdose
lithium with pyrroloquinoline quinone in an Alzheimer's disease mouse model. Neurobiol
Aging 35: 2736-2745. [Crossref]
13. Nakano M, Murayama
Y, Hu L, Ikemoto K, Uetake T et al. (2016) Effects of Antioxidant Supplements
(BioPQQ™) on Cerebral Blood Flow and Oxygen Metabolism in the Prefrontal
Cortex. Adv Exp Med Biol 923: 215-222. [Crossref]
14. Chowanadisai W,
Bauerly KA, Tchaparian E, Wong A, Cortopassi GA et al. (2010) Pyrroloquinoline
quinone stimulates mitochondrial biogenesis through cAMP response
element-binding protein phosphorylation and increased PGC-1alpha expression. J
Biol Chem 285: 142-152. [Crossref]
15. Lee JW, Lee YK, Yuk
DY, Choi DY, Ban SB et al. (2008) Neuro-inflammation induced by
lipopolysaccharide causes cognitive impairment through enhancement of
beta-amyloid generation. J Neuroinflammation 5: 37. [Crossref]
16. Zakaria A, Hamdi N,
Abdel-Kader RM (2016) Methylene Blue Improves Brain Mitochondrial ABAD
Functions and Decreases Aβ in a Neuroinflammatory Alzheimer's Disease Mouse
Model. Mol Neurobiol 53: 1220-1228. [Crossref]
17. Mostafa AO,
Abdel-Kader RM, Heikal OA (2018) Enhancement of cognitive functions by rice
bran extract in a neuroinflammatory mouse model via regulation of PPARγ. J
Functional Foods 48: 314-321.
18. Zhang Y, Feustel
PJ, Kimelberg HK (2006) Neuroprotection by pyrroloquinoline quinone (PQQ) in
reversible middle cerebral artery occlusion in the adult rat. Brain Res 1094:
200-206. [Crossref]
19. Lu H, Shen J, Song
X, Ge J, Cai R et al. (2015) Protective Effect of Pyrroloquinoline Quinone
(PQQ) in Rat Model of Intracerebral Hemorrhage. Cell Mol Neurobiol 35:
921-930. [Crossref]
20. Bevins RA, Besheer
J (2006) Object recognition in rats and mice: a one-trial
non-matching-to-sample learning task to study 'recognition memory'. Nat
Protoc 1: 1306-1311. [Crossref]
21. Arai K, Matsuki N,
Ikegaya Y, Nishiyama N (2001) Deterioration of spatial learning performances in
lipopolysaccharide-treated mice. Jpn J Pharmacol 87: 195-201. [Crossref]
22. Morris R (1984)
Developments of a water-maze procedure for studying spatial learning in the
rat. J Neurosci Methods 11: 47-60. [Crossref]
23. Nunez J (2008)
Morris Water Maze Experiment. J Vis Exp 897. [Crossref]
24. Vorhees CV,
Williams MT (2006) Morris water maze: procedures for assessing spatial and
related forms of learning and memory. Nat Protoc 1: 848-858. [Crossref]
25. Ormerod BK, Hanft
SJ, Asokan A, Haditsch U, Lee SW et al. (2013) PPARγ activation prevents
impairments in spatial memory and neurogenesis following transient illness. Brain
Behav Immun 29: 28-38. [Crossref]
26. Dehkordi NG,
Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M (2015) Omega-3 fatty acids
prevent LPS-induced passive avoidance learning and memory and CaMKII-α gene
expression impairments in hippocampus of rat. Pharmacol Rep 67: 370-375.
[Crossref]
27. Song X, Zhou B,
Zhang P, Lei D, Wang Y et al. (2016) Protective Effect of Silibinin on Learning
and Memory Impairment in LPS-Treated Rats via ROS-BDNF-TrkB Pathway. Neurochem
Res 41: 1662-1672. [Crossref]
28. Zhang L, Liu J,
Cheng C, Yuan Y, Yu B et al. (2012) The neuroprotective effect of
pyrroloquinoline quinone on traumatic brain injury. J Neurotrauma 29:
851-864. [Crossref]
29. Sawmiller D, Li S,
Mori T, Habib A, Rongo D et al. (2017) Beneficial effects of a
pyrroloquinolinequinone-containing dietary formulation on motor deficiency,
cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer's
disease. Heliyon 3: e00279. [Crossref]
30. Kim J, Kim J, Shim
J, Lee S, Kim J et al. (2013) Licorice-derived dehydroglyasperin C increases
MKP-1 expression and suppresses inflammation-mediated neurodegeneration. Neurochem
Int 63: 732-740. [Crossref]
31. Rosenberger K,
Derkow K, Dembny P, Kruger C, Schott E et al. (2014) The impact of single and
pairwise Toll-like receptor activation on neuroinflammation and neurodegeneration.
J Neuroinflammation 11: 166. [Crossref]
32. Tegenge MA,
Rajbhandari L, Shrestha S, Mithal A, Hosmane S et al. (2014) Curcumin protects
axons from degeneration in the setting of local neuroinflammation. Exp
Neurol 253: 102-110. [Crossref]
33. Wang Q, Chu CH,
Qian L, Chen SH, Wilson B et al. (2014) Substance P exacerbates dopaminergic
neurodegeneration through neurokinin-1 receptor-independent activation of
microglial NADPH oxidase. J Neurosci 34: 12490-12503. [Crossref]
34. Lykhmus O, Mishra N, Koval L, Kalashnyk O, Gergalova G et al. (2016) Molecular
Mechanisms Regulating LPS-Induced Inflammation in the Brain. Front Mol
Neurosci 9: 19. [Crossref]
35. Zhang Q, Ding M,
Cao Z, Zhang J, Ding F et al. (2013) Pyrroloquinoline quinine protects rat
brain cortex against acute glutamate-induced neurotoxicity. Neurochem Res
38: 1661-1671. [Crossref]
36. Qin J, Wu M, Yu S,
Gao X, Zhang J et al. (2015) Pyrroloquinoline quinone-conferred neuroprotection
in rotenone models of Parkinson's disease. Toxicol Lett 238: 70-82. [Crossref]
37. Lu J, Chen S, Shen
M, He Q, Zhang Y et al. (2018) Mitochondrial regulation by pyrroloquinoline
quinone prevents rotenone-induced neurotoxicity in Parkinson's disease models. Neurosci
Lett 687: 104-110. [Crossref]
38. Suliman HB,
Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA (2003) Lipopolysaccharide
stimulates mitochondrial biogenesis via activation of nuclear respiratory
factor-1. J Biol Chem 278: 41510-41518. [Crossref]
39. Suliman HB, Sweeney
TE, Withers CM, Piantadosi CA (2010) Co-regulation of nuclear respiratory
factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial
biogenesis. J Cell Sci 123: 2565-2575. [Crossref]
40. Suliman HB,
Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA (2004) Lipopolysaccharide
induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc
Res 64: 279-288. [Crossref]
41. Qian J, Chen S,
Huang Y, Shi X, Liu C (2013) PGC-1α regulates hepatic hepcidin expression and
iron homeostasis in response to inflammation. Mol Endocrinol 27:
683-692. [Crossref]
42. Smith JA, Stallons
LJ, Collier JB, Chavin KD, Schnellmann RG (2015) Suppression of mitochondrial
biogenesis through toll-like receptor 4-dependent mitogen-activated protein
kinase kinase/extracellular signal-regulated kinase signaling in
endotoxin-induced acute kidney injury. J Pharmacol Exp Ther 352:
346-357. [Crossref]
43. Bullon P, Roman-Malo L, Marin-Aguilar F, Alvarez-Suarez JM, Giampieri F et
al. (2015)
Lipophilic antioxidants prevent lipopolysaccharide-induced mitochondrial dysfunction
through mitochondrial biogenesis improvement. Pharmacol Res 91: 1-8. [Crossref]
44. Sun W, Zheng Y, Lu
Z, Cui Y, Tian Q et al. (2014) Overexpression of S100A7 protects LPS-induced
mitochondrial dysfunction and stimulates IL-6 and IL-8 in HaCaT cells. PLoS
One 9: e92927. [Crossref]
45. Denis MC,
Desjardins Y, Furtos A, Marcil V, Dudonne S et al. (2015) Prevention of
oxidative stress, inflammation and mitochondrial dysfunction in the intestine
by different cranberry phenolic fractions. Clin Sci (Lond) 128: 197-212.
[Crossref]
46. Wang LF, Yang GQ,
Yang S, Yang GY, Li M et al. (2015) Alteration of factors associated with
hepatic gluconeogenesis in response to acute lipopolysaccharide in dairy goat. J
Anim Sci 93: 2767-2777. [Crossref]
47. Correa F, Ljunggren
E, Patil J, Wang X, Hagberg H et al. (2013) Time-dependent effects of systemic
lipopolysaccharide injection on regulators of antioxidant defence Nrf2 and
PGC-1α in the neonatal rat brain. Neuroimmunomodulation 20: 185-193. [Crossref]
48. Tanaka K, Tanaka M,
Takegaki J, Fujino H (2016) Preventive effects of electrical stimulation on
inflammation-induced muscle mitochondrial dysfunction. Acta Histochem
118: 464-470. [Crossref]
49. Khedr LH, Nassar
NN, Rashed L, El-Denshary ED, Abdel-Tawab AM (2019) TLR4 signaling modulation
of PGC1-alpha mediated mitochondrial biogenesis in the LPS-Chronic mild stress
model: Effect of fluoxetine and pentoxiyfylline. Life Sci 239: 116869. [Crossref]
50. Bauerly KA, Storms
DH, Harris CB, Hajizadeh S, Sun MY et al. (2006) Pyrroloquinoline quinone
nutritional status alters lysine metabolism and modulates mitochondrial DNA
content in the mouse and rat. Biochim Biophys Acta 1760: 1741-1748. [Crossref]
51. Stites T, Storms D,
Bauerly K, Mah J, Harris C et al. (2006) Pyrroloquinoline quinone modulates
mitochondrial quantity and function in mice. J Nutr 136: 390-396. [Crossref]
52. Kuo YT, Shih PH,
Kao SH, Yeh GC, Lee HM (2015) Pyrroloquinoline Quinone Resists
Denervation-Induced Skeletal Muscle Atrophy by Activating PGC-1α and
Integrating Mitochondrial Electron Transport Chain Complexes. PLoS One
10: e0143600. [Crossref]
53. Singh AK, Pandey
SK, Saha G, Gattupalli NK (2015) Pyrroloquinoline quinone (PQQ) producing
Escherichia coli Nissle 1917 (EcN) alleviates age associated oxidative stress
and hyperlipidemia, and improves mitochondrial function in ageing rats. Exp
Gerontol 66: 1-9. [Crossref]
54. Cheng Q, Chen J, Guo H, Lu JL, Zhou J et al. (2021)
Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced
Parkinson’s disease model via AMPK activation. Acta Pharmacol Sin 42:
665-678. [Crossref]
55. Adami PVM, Quijano C, Magnani N, Galeano P, Evelson P et al. (2017) Synaptosomal
bioenergetic defects are associated with cognitive impairment in a transgenic
rat model of early Alzheimer's disease. J Cereb Blood Flow Metab 37:
69-84. [Crossref]
56. Wang Z, Chen GQ, Yu
GP, Liu CJ (2014) Pyrroloquinoline quinone protects mouse brain endothelial
cells from high glucose-induced damage in vitro. Acta Pharmacol Sin 35:
1402-1410. [Crossref]
57. Zhang Q, Zhang J, Jiang C, Qin J, Ke K et al. (2014) Involvement of ERK1/2 pathway in neuroprotective effects of pyrroloquinoline quinine against rotenone-induced SH-SY5Y cell injury. Neuroscience 270: 183-191. [Crossref]
58. Bauerly K, Harris C, Chowanadisai W, Graham J, Havel PJ et al. (2011) Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One 6: e21779. [Crossref]
