Real World Patterns of PSA Response and Survival with Abiraterone and Enzalutamide in Metastatic Castrate Resistant Prostate Cancer (mCRPC)
A B S T R A C T
Background: We investigated our institution’s mCRPC enzalutamide or abiraterone patients examining PSA responses and impact of sequencing of these drugs.
Methods: All enzalutamide / abiraterone mCRPC patients (2011-2018) were included. Rates of PSA >50% response (PSA50) were compared. Time to treatment failure (TTF) and overall survival (OS) was analysed as per lines of previous therapy and timing of chemotherapy.
Results: 363 patients included (Enza n=236, Abi n=127), with 15.6 months median follow-up. PSA50 was greater in enzalutamide group (58% vs 31% p<0.0005) but TTF was similar for both groups (median Abi 4.2m vs Enza 6m, p=0.965). There was no significant median OS difference between the groups (Enza 13.8m vs Abi 12.5m p=0.065). Number of lines of prior therapy (p=0.735) or timing of chemotherapy before or after Abi/Enza (p=0.21) had no significant OS impact.
Conclusion: Enzalutamide showed higher PSA50 response than abiraterone. Previous lines of therapy or sequencing of chemotherapy with abiraterone / enzalutamide showed no significant survival differences indicating no detriment in either treatment sequence.
Keywords
Metastatic prostate cancer, enzalutamide, abiraterone, androgen receptor targeted agents, treatment sequence
Introduction
There are approximately 11,500 prostate cancer-related deaths every year in the United Kingdom (UK) [1]. The majority of these are due to metastatic disease. After initial androgen deprivation therapy (ADT), metastatic castrate resistant prostate cancer (mCRPC) is defined as progression of prostate cancer despite castrate (less than 50ng/L) levels of testosterone. Novel androgen receptor targeted agents (ARTA) including abiraterone and enzalutamide are in widespread use in mCRPC. Abiraterone- given alongside prednisolone (here onwards called abiraterone or Abi) is an androgen biosynthesis inhibitor of CYP17A1- a key enzyme in extra-testicular and testicular androgen formation [2]. Enzalutamide (here onwards called Enza) is an androgen-receptor-signalling inhibitor that inhibits androgen receptor translocation, DNA binding and coactivator recruitment [3].
Both these ARTA drugs are in widespread clinical use in the treatment of mCRPC based on several trials demonstrating overall (OS) and progression free survival (PFS) advantage in patients previously treated with docetaxel chemotherapy and pre-chemotherapy groups respectively [4-8]. Chopra et al. indirectly compared abiraterone and enzalutamide using Bayesian modelling from these main trial results (listed above) showing better prostate-specific antigen (PSA) response and PFS for Enza but no OS difference [9]. There have been no randomised published head to head clinical studies comparing the efficacy and toxicity of both Abi and Enza drugs in the pre and post chemotherapy setting. As such there are no indications or guidelines for choosing between one from the other. We also have limited data suggesting superior sequencing of ARTA, chemotherapy and novel agents [10, 11]. George et al. looked at OS and economic burden comparing abiraterone and enzalutamide in 3174 chemo-naïve mCRPC patients. This showed similar median OS (Enza 29m and Abi 26m) with reduced overall health care costs for the enzalutamide group [12]. In this study we aim to look at our population of mCRPC patients treated with abiraterone or enzalutamide and examine the impact of the sequencing of either ARTA therapy on the patients’ clinical outcomes.
Materials and Methods
I Study Design and Population
We have collected single institution retrospective data from our ARIA systemic anti-cancer prescribing system for all patients treated at the Royal Cornwall Hospital Oncology centre with either abiraterone or enzalutamide for mCRPC from September 2011 to October 2017. Exclusion criteria were patients treated with abiraterone or enzalutamide in the hormone sensitive metastatic prostate cancer setting in clinical trials. The study had full trust approval- Trust ID 520.
II Data Collection
Patient characteristics were collected including age, number of previous lines of treatment, diagnosis date, Gleason Grade, PSA at treatment initiation, PSA at 4 weeks on treatment, PSA nadir count, treatment cessation date and survival. Patients were excluded if they received Abi or Enza in the metastatic hormone sensitive prostate cancer (mHSPC) setting.
III Outcome Measures
i Primary Outcome
A. PSA response to treatment- any PSA response (PSAr) and >50% PSA reduction from baseline (PSA50).
B. Overall survival (OS)
a. From time of initiation of ARTA treatment.
b. From time of initial prostate cancer diagnosis and the OS impact of ARTA given pre or post docetaxel chemotherapy.
c. Impact of how many previous lines of therapy before ARTA therapy affected OS.
ii Secondary Outcomes
A. Time to clinical treatment failure (TTF)- defined as the time treatment was stopped by clinician.
B. Time to biochemical failure (TBF)- defined as PSA 25% above nadir count or +2ng/L.
IV Statistical Analysis
For normally distributed data continuous outcomes were presented as means or medians and standard deviations, categorical data were presented as frequencies and proportions. Kaplan Meier and Log-Rank analysis of overall survival and time to treatment failure were performed. Chi-squared testing was performed on the rates of PSA response between the groups and proportions of patients receiving treatment for more than 6 months- significance value was set at p<0.05. Waterfall plots for the individual drugs pre and post chemotherapy use with PSA levels were also performed. Data were analysed with IBM SPSS statistics version 254.0 and R-statistics packages.
Results
In total 363 patients (Enza n=236, Abi n=127) were included with a median follow-up of 15.6 months. Patient characteristics are summarised in (Table 1). Median starting PSA was not significantly different between groups: Abi group 81 ng/L vs Enza group 59 ng/L (p=0.06). Biopsies had not been taken in a significant proportion of patients in both groups: 47% in Enza vs 62% in Abi group.
Table 1: Baseline characteristics for Abiraterone and Enzalutamide patients.
|
|
Enzalutamide |
Abiraterone |
p values |
|
Number |
236 |
127 |
|
|
Median age (range) Years |
76 (57 - 93) |
76 (52 - 89) |
p = 0.310 |
|
Gleason prognostic group distribution: 1 2 3 4 5 Not recorded |
6% 6% 8% 12% 21% 47% |
8% 6% 4% 6% 14% 62% |
p = 0.576 p = 0.994 p = 0.136 p = 0.075 p = 0.091 p = 0.007 |
|
Previous lines of treatment 1 2 3 4 5 |
1% 32% 55% 10% 2% |
2% 35% 55% 8% |
p = 0.235 p = 0.622 p = 0.964 p = 0.485 |
|
Median starting PSA |
59 (0.03-9537) |
81 (2.1-1834) |
p = 0.364 |
|
Proportion chemotherapy-naïve |
79% |
89% |
p = 0.075 |
Patients were heavily pre-treated, with 67% of Enza and 63% of Abi patients having had 3 or more lines of previous therapy. The majority of patients were docetaxel naïve: 79% in Enza and 89% in Abi group.
I PSA Responses
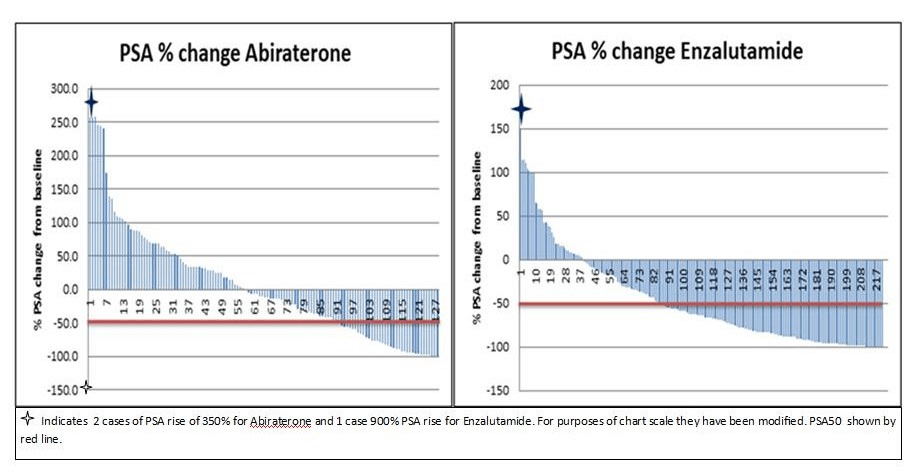
Significantly more PSA responses were seen in the Enza group compared to Abi with PSA50 = 58% Enza vs 31% Abi (p<0.0005). PSAr, again, was more likely in the Enza group (Enza 81% vs Abi 56%- p<0.0005). Maximum PSA changes from ARTA starting level are displayed in the waterfall charts below (Figure 1). PSA responses were higher in the pre compared to post-chemotherapy group which is in line with the previously mentioned COU PREVAIL and AFFIRM studies (Table 2) [5-8].
Table 2: Summary of PSA responses Abiraterone and Enzalutamide groups.
|
|
Enza (n=236) |
Abi (n=127) |
p-value |
|
Proportion with PSA response (any) |
80.1% |
56.7% |
P < 0.001 |
|
Proportion with PSA response >50% |
58.5% |
30.9% |
P < 0.001 |
|
|
|
|
|
|
Pre-Docetaxel |
185 |
90 |
P = 0.111 |
|
PSA response Any PSA50 |
152 111 |
52 32 |
P < 0.001 P < 0.001 |
|
|
|
|
|
|
Post-Docetaxel |
51 |
37 |
P = 0.111 |
|
PSA response Any PSA50 |
37 26 |
20 7 |
P = 0.073 P = 0.002 |
Figure 1: Waterfall chart showing maximal PSA change from baseline in Abiraterone and Enzalutamide groups.
II Survival Results
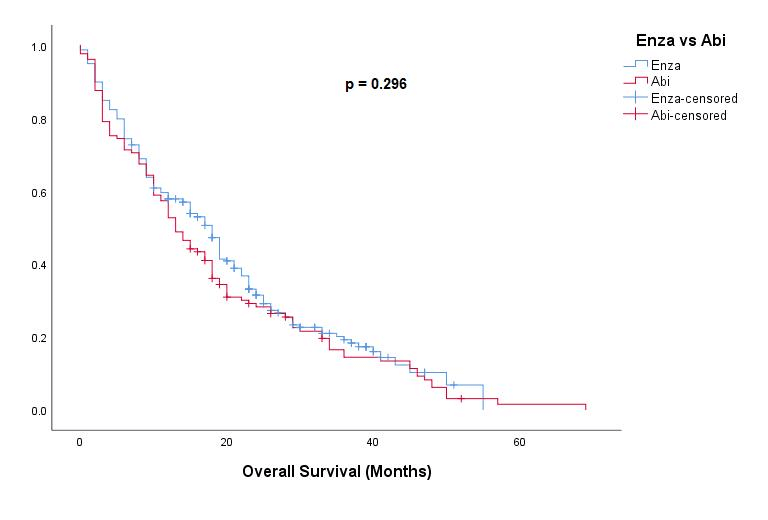
There was no significant difference in median OS from the start of ARTA treatment between the two drug groups: Enza 13.8m vs Abi 12.5m, (p=0.065) (Figure 2). When comparing survival vs death by different treatments there were more Enza patients alive vs Abi patients (23% vs 8%) p<0.0001.
Figure 2: Kaplan Meier survival curves for Overall survival comparing Enza vs Abi patient groups.
III Effect of Line of Therapy
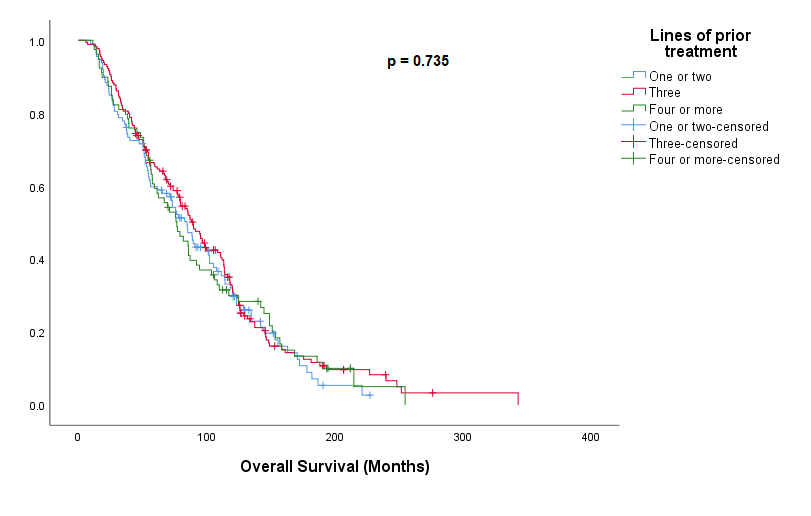
The line of mCRPC treatment that ARTA therapy was started on did not influence OS when assessed from date of prostate cancer diagnosis. Median OS of 85m - <3 lines, 90m – 3 lines or 77m for >3 lines of prior therapy (p=0.735) (Figure 3).
Figure 3: Kaplan Meier showing Overall Survival with ARTA therapy based on number of previous lines of therapy.
IV Sequencing of ARTA with Chemotherapy and OS
In our patients who received chemotherapy (n= 146) there was no significant difference in OS from date of their cancer diagnosis whether the ARTA was given before chemotherapy median OS 85.0 months (95 % CI 64.4-105.7m) vs ARTA given after chemotherapy 75.8months (95 % CI 66.0 – 85.6) (p=0.21) (Figure 4).
Figure 4: Kaplan-Meier showing overall survival based on delivery of ARTA therapy before or after docetaxel chemotherapy.
Analysis of OS from diagnosis date looking at the complete patient group (including those patients who did not receive chemotherapy) shows that patients median OS for the group without any prior chemotherapy was higher than those who had prior chemotherapy (p=0.043).
V Treatment Failure Results
There was no significant difference seen in time to clinical treatment failure: Abi 4.2months vs Enza 6months (p=0.204) or time to biochemical failure: Abi 3.5months vs Enza 4.4months (p=0.965). The clinical treatment failure times were similar for the chemotherapy naïve (Abi 10months vs Enza 8months, p=0.60) and post chemotherapy groups (Abi 6months vs Enza 5months, p=0.191).
Discussion
In this study we have showed better PSA responses in the enzalutamide group in all settings, but this has not led to significant differences in OS, time to biochemical or clinical treatment failure. The higher PSA responses for enzalutamide are in keeping with findings from previous comparisons of studies by Chopra et al. as discussed earlier and this is now confirmed in our real-world data [9].
In our cohort we had a high proportion of patients without a biopsy which makes it difficult for comparison of Gleason grading between treatment groups. Many patients had been heavily pre-treated with two-thirds having had three or more lines of previous therapy. This may have some impact on subsequent time to treatment failure but the sequencing of treatment with ARTA did not impact on overall survival from cancer diagnosis in the mCRPC setting in our cohort.
A large proportion of patients were given ARTA pre-chemotherapy. This may change over time with increased early docetaxel chemotherapy being used in mHSPC as per the CHAARTED and STAMPEDE study results [13, 14]. Our results demonstrated no detriment to receiving ARTA before chemotherapy as OS was similar in both pre-and-post docetaxel populations. This could be subject to selection bias as patients with higher risk disease or greater symptom burden may have been started on docetaxel earlier in their pathway. It does suggest that patients having ARTA therapy before chemotherapy are not being disadvantaged.
The position of Abi and Enza in the sequence of mCRPC may change with the LATITUDE, STAMPEDE and ENZAMET study results showing benefits similar to chemotherapy in the mHSPC setting [13-16]. This may move the decision to use these drugs in metastatic prostate cancer earlier in the treatment pathway and indeed into the non-metastatic castrate resistant setting: EMBARK, PROSPER, IMAAGEN trials [17-19]. Quality of life in mCRPC is a key factor in treatment selection and selecting the correct treatment for the patient at the correct point in their cancer journey can have a marked impact on their quality of life [20, 21].
I Limitations
This is a retrospective dataset and as such do not have data on significant prognostic markers such as bone pain or quality of life data. We have a significant proportion of patients without a biopsy and hence no Gleason grade. Despite these limitations we feel this is a significant real-world population of mCRPC patients with good PSA and clinical follow up.
II Learning Points
We have shown that clinician choice of drug for a patient with mCRPC does not have a negative impact on OS. Delaying the use of chemotherapy in mCRPC patients may improve patient quality of life and our data would support ARTA use before chemotherapy.
Conclusion
This study showed greater PSA response rates for enzalutamide compared to abiraterone. We saw no major difference in OS when ARTA was started before or after chemotherapy or with number of lines of prior therapy with again no evidence of treatment sequence affecting OS outcome in mCRPC. This indicated it may be reasonable to use ARTA therapy before chemotherapy in the patient’s treatment sequence.
Disclaimer
None.
Conflicts of Interest
John McGrane has received travel and speaking honoraria from Astellas and speaking honoraria from Jannsen-Cilag, Deborah Victor has received travel honorarium from Astellas, Michael Rowe has received a travel honorarium from Astellas. All other authors report no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 29, Jun 2020Accepted: Wed 08, Jul 2020
Published: Mon 13, Jul 2020
Copyright
© 2023 John McGrane. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.07.11
Author Info
Michael Rowe Stuart Walter Ayesha Hidayat Hannah Donkers Andrew Browne Timothy Norris Adam Pollard Deborah Victor John McGrane
Corresponding Author
John McGraneConsultant Oncologist, Sunrise Oncology Centre, Royal Cornwall Hospital, Truro, UK
Figures & Tables
Table 1: Baseline characteristics for Abiraterone and Enzalutamide patients.
|
|
Enzalutamide |
Abiraterone |
p values |
|
Number |
236 |
127 |
|
|
Median age (range) Years |
76 (57 - 93) |
76 (52 - 89) |
p = 0.310 |
|
Gleason prognostic group distribution: 1 2 3 4 5 Not recorded |
6% 6% 8% 12% 21% 47% |
8% 6% 4% 6% 14% 62% |
p = 0.576 p = 0.994 p = 0.136 p = 0.075 p = 0.091 p = 0.007 |
|
Previous lines of treatment 1 2 3 4 5 |
1% 32% 55% 10% 2% |
2% 35% 55% 8% |
p = 0.235 p = 0.622 p = 0.964 p = 0.485 |
|
Median starting PSA |
59 (0.03-9537) |
81 (2.1-1834) |
p = 0.364 |
|
Proportion chemotherapy-naïve |
79% |
89% |
p = 0.075 |
Table 2: Summary of PSA responses Abiraterone and Enzalutamide groups.
|
|
Enza (n=236) |
Abi (n=127) |
p-value |
|
Proportion with PSA response (any) |
80.1% |
56.7% |
P < 0.001 |
|
Proportion with PSA response >50% |
58.5% |
30.9% |
P < 0.001 |
|
|
|
|
|
|
Pre-Docetaxel |
185 |
90 |
P = 0.111 |
|
PSA response Any PSA50 |
152 111 |
52 32 |
P < 0.001 P < 0.001 |
|
|
|
|
|
|
Post-Docetaxel |
51 |
37 |
P = 0.111 |
|
PSA response Any PSA50 |
37 26 |
20 7 |
P = 0.073 P = 0.002 |

References
- CRUK Prostate Cancer Statistics. Cancer Research UK.
- Gerhardt Attard, Alison H M Reid, Roger A'Hern, Christopher Parker, Nikhil Babu Oommen et al. (2009) Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 27: 3742-3748. [Crossref]
- Jack Schalken, John M Fitzpatrick (2016) Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int 117: 215-225. [Crossref]
- Johann S de Bono, Christopher J Logothetis, Arturo Molina, Karim Fizazi, Scott North, Luis Chu et al. (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364: 1995-2005. [Crossref]
- Charles J Ryan, Matthew R Smith, Karim Fizazi, Fred Saad, Peter F A Mulders et al. (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16: 152-160. [Crossref]
- Howard I Scher, Karim Fizazi, Fred Saad, Mary Ellen Taplin, Cora N Sternberg et al. (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367: 1187-1197. [Crossref]
- Tomasz M Beer, Andrew J Armstrong, Dana E Rathkopf, Yohann Loriot, Cora N Sternberg et al. (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371: 424-433. [Crossef]
- S Salem, M Komisarenko, N Timilshina, L Martin, R Grewal et al. (2017) Impact of Abiraterone Acetate and Enzalutamide on Symptom Burden of Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer. Clin Oncol 29: 601-608. [Crossref]
- Akhil Chopra, Mina Georgieva, Gilberto Lopes, Chong Ming Yeo, Benjamin Haaland (2017) Abiraterone or Enzalutamide in advanced castration resistant prostate cancer: An indirect comparison. Prostate 77: 639-646. [Crossref]
- Souhil Lebdai, Victor Basset, Julien Branchereau, Alexandre de La Taille, Vincent Flamand et al. (2016) What do we know about treatment sequencing of abiraterone, enzalutamide and chemotherapy in metastatic castration-resistant prostate cancer? World J Urol 34: 617-624. [Crossref]
- A. Angelergues, A.J. Birtle, A.C. Hardy Bessard, O. Caffo, S. Le Moulec et al. (2016) Efficacy of cabazitaxel, abiraterone, enzalutamide and docetaxel sequence in men with metastatic castration-resistant prostate cancer (mCRPC) in real life practice: the multinational, retrospective, observational CATS study. Ann Oncol 27: 243-265.
- Daniel George AUA 2019 Survival Rates and Economic Outcomes in Chemotherapy-Naïve Metastatic Castrate Resistant Prostate Cancer Patients treated with Abiraterone Acetate or Enzalutamide.
- Christopher J Sweeney, Yu Hui Chen, Michael Carducci, Glenn Liu, David F Jarrard et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373: 737-746. [Crossref]
- Nicholas D James, Matthew R Sydes, Noel W Clarke, Malcolm D Mason, David P Dearnaley et al. (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387: 1163-1177. [Crossref]
- Ian D Davis, Andrew J Martin, Martin R Stockler, Stephen Begbie, Kim N Chi et al. (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381: 121-131. [Crossref]
- Karim Fizazi, NamPhuong Tran, Luis Fein, Nobuaki Matsubara, Alfredo Rodriguez Antolin et al. (2017) Abiraterone plus prednisone in metastatic, castrate-sensitive prostate cancer. N Engl J Med 377: 352-360. [Crossref]
- Maha Hussain, Karim Fizazi, Fred Saad, Per Rathenborg, Neal Shore et al. (2018) Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Eng J Med 378: 2465-2474. [Crossref]
- Safety and Efficacy Study of Enzalutamide Plus Leuprolide in Patients with Nonmetastatic Prostate Cancer (EMBARK) NCT02319837.
- Charles J Ryan, E David Crawford, Neal D Shore, Willie Underwood 3rd, Mary Ellen Taplin et al. (2018) The IMAAGEN Study: Effect of Abiraterone Acetate and Prednisone on Prostate-Specific Antigen and Radiographic Disease Progression in Patients with Non-Metastatic Castration-Resistant Prostate Cancer. J Urol 200: 344-352. [Crossref]
- Daniel J Khalaf, Katherine Sunderland, Bernhard J Eigl, Christian K Kollmannsberger, Nikita Ivanov et al. (2019) Health related quality of life for abiraterone plus prednisolone versus Enzalutamide in patients with metastatic castration resisitant prostate cancer: results from a phase II randomised trial. Eur Urol 75: 940-947. [Crossref]
- V Jenkins, I Solis Trapala, H Payne, M Mason, L Fallowfield et al. (2019) Treatment Experiences, Information Needs, Pain and Quality of Life in Men with Metastatic Castrate-resistant Prostate Cancer: Results from the EXTREQOL Study. Clin Oncol 31: 99-107. [Crossref]
