Regenerative and Endoscopic Treatment of Complex Recurrent Fistula in Ano: When Technology Supports Clinical Treatment
A B S T R A C T
Background: Recurrent anal fistulas present a challenge to surgeons due to the high risk of post-operative incontinence caused by repeated surgery. The correct identification of the anatomy of the main and secondary fistula tracts and the individuation of abscess cavities are fundamental for correct treatment. Intraoperative endoscopic evaluation and the complete destruction of the fistula pathway can be achieved through video-assisted anal fistula treatment (VAAFT). Furthermore, the injection of human autologous Microfractured Adipose Tissue (MFAT) processed by a Lipogems® device can be used as both a bulking agent and a regenerative technique.
Methods: A combined approach of VAAFT plus Microfractured Adipose Tissue Graft (MFAT) is proposed in order to treat recurrent and complex fistula in ano.
Results: Three cases treated with a combination of VAAFT and MFAT grafts are described. All cases had undergone multiple interventions at the perianal level over a period ranging from 1 to 15 years. One case certainly failed due to poor patient compliance, but in the remaining two cases, the patients made a complete recovery with the disappearance of symptoms over a follow-up period of one to two years.
Conclusion: The combination of video-assisted anal fistula treatment and injection of human autologous microfractured adipose tissue may be a valid, safe and feasible therapeutic option. MFAT injections are more effective in promoting tissue regeneration than simply “filling” the fistula tract and are common practice also in the treatment of Crohn’s Disease due to the immunomodulatory power of mesenchymal cells.
Keywords
Anal fistula, VAAFT, Lipogems®, regenerative surgery, autologous fat graft, microfractured adipose tissue
Introduction
An anal fistula is an abnormal pathway between the anorectal area and perianal skin. It is the consequence of acute infections that have not healed, such as abscesses, but may also be the result of a complication of Crohn’s disease, tuberculosis, malignancy and trauma. It affects 9 in 100,000 people per year in Western populations [1]. Anal fistulas can be classified as simple or complex: simple fistulas involve only the internal sphincter (inter-sphincteric) or have a superficial trans-sphincteric course; complex fistulas, on the other hand, traverse more than 30% of the external sphincter or the anterior sphincter in female patients. Fistulas with multiple tracts, recurrent fistulas, and finally, those occurring after local irradiation or associated to Crohn’s disease are also classified as complex [2].
Recurrent anal fistulas present a challenge to surgeons due to the high risk of post-operative incontinence caused by repeated surgery. The correct identification of the internal opening, the identification of the course of the main and secondary fistula tracts and the individuation of abscess cavities are fundamental for correct treatment. This can be achieved through a thorough pre-operative examination by means of an MRI and, in particular, with a thorough intraoperative endoscopic evaluation.
An intraoperative endoscopic evaluation can be performed using video-assisted anal fistula treatment (VAAFT), a mininvasive technique introduced in 2006 by Dr.med. Meinero to treat anal fistulae, which can be particularly useful in the treatment of complex and post-traumatic cases [3, 4].
The complete eradication of infections and the hermetic closure of the internal opening are essential for preventing the passage of faecal material through the fistula pathway, as well as for healing and preventing recurrence [5]. In the last two decades, various innovative techniques have been proposed in anal fistula surgery: Fistula Laser Closure (FiLaC), ligation of the intersphincteric fistula tract (LIFT) and the injection of bulking agents [6-9]. The injection of human autologous Microfractured Adipose Tissue (MFAT) processed by Lipogems® can be used as both a bulking agent and a regenerative technique and has already been adopted in several surgical fields, such as orthopaedic surgery, wound healing, faecal incontinence and anal fistula surgery [10-14]. In this paper a combined approach of VAAFT plus MFAT graft is proposed in order to treat recurrent and complex fistula in ano.
Materials and Methods
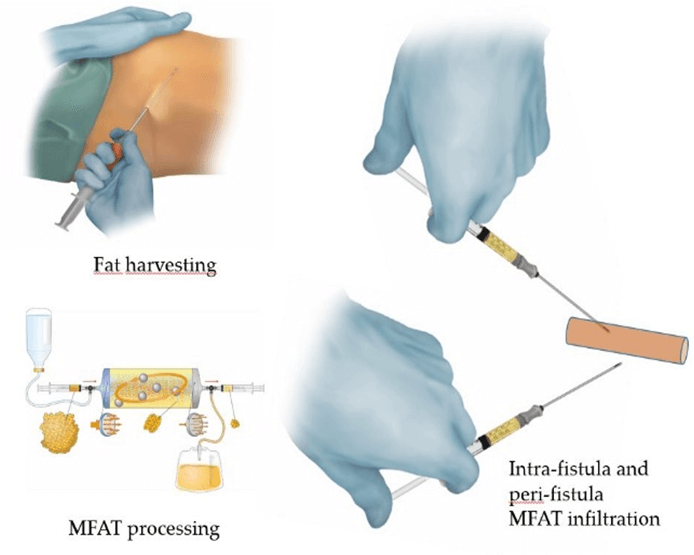
The first phase of the intervention is the collection of adipose tissue. Normally the donor sites are the abdomen, the hips, the trochanteric regions and the inside of the thighs. This phase is performed paying extreme attention to antisepsis and therefore must precede the procedure in the anal site. Liposuction is performed in a lithotomy position, under general anaesthesia after infiltrating the donor sites with a 0.9% NaCl solution with adrenaline in a concentration of one part per thousand. Sampling is performed using a 20 cc VacLok® Vacuum Pressure Syringe and a 13G Lipogems® aspiration cannula.
The second phase is the micro-fragmentation of the aspirated adipose tissue. Micro-fragmentation is achieved using the 240ml Lipogems® device (Figure 1). The Microfractured Adipose Tissue (MFAT) processed by Lipogems® is collected in 10cc Luer lock syringes and left to settle in an upright position.
Figure 1: Lipogems® device for the microfragmentation of adipose tissue (MFAT).
1: Spike for wash solution bag; 2: Soft drip chamber; 3: Washing line inlet clamp; 4: Washing line; 5: Input clamp; 6: Lipoaspirate loading valve; 7: Blue head (inlet filter); 8: Processing unit; 9: Gray head (outlet filter); 10: MFAT extraction valve; 11: Clamp discharge line; 12: Discharge line; 13: Discharge bag connection clamp; 14: Discharge bag connection; 15: Discharge bag clamp; 16: Discharge bag.
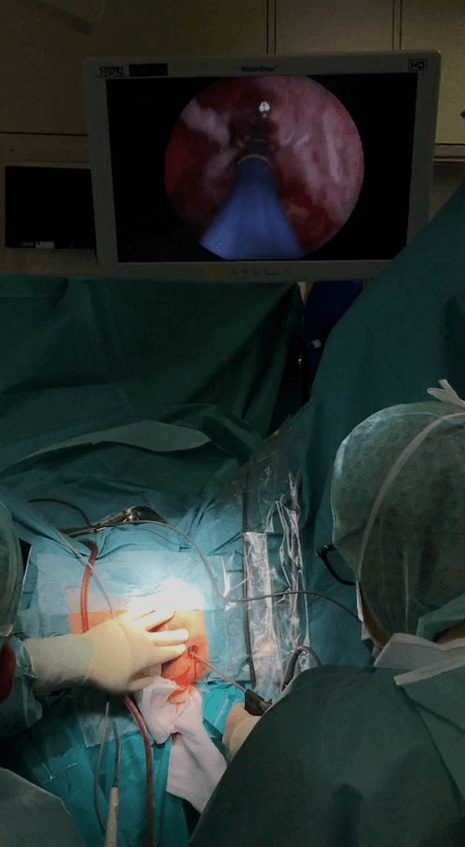
The third phase of the intervention consists of VAAFT diagnostic and operative procedures. The fistuloscope is connected to a washing fluid of glycine and 1% mannitol solution. The diagnostic phase starts by introducing the fistuloscope through the external opening of the fistula and identifying the main pathway, as well as any possible secondary tracts or abscess cavities. Normally the fistula “anatomy” can be traced up to the end of the fistula tract, which is usually the internal fistula opening. The operative phase involves the complete destruction of the fistula pathway, under fibroscopic control, with the passage of a unipolar electrode through the fistuloscope (Figure 2). Any residual necrotic material is removed with the aid of an endo-brush and the internal orifice is subsequently sutured under direct vision with simple stitches applied to the internal opening or, in 15% of cases, with a linear stapler [4]. In addition, an advancement flap can be made for the safer closure of the internal orifice.
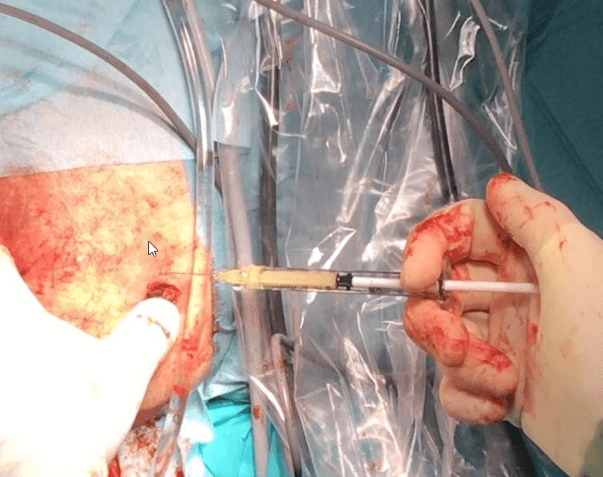
The fourth and last phase of the operation consists of the transplantation of MFAT both inside the fistulous tract sanitised with VAAFT and in the peri-fistular tissues using a 1cc Luer lock syringe and 20G cannulas or needles (Figures 3 & 4). The external fistular orifice is usually sutured to avoid the expulsion of the deposited adipose tissue.
Figure 2: VAAFT procedure: operating theatre view.
Figure 3: Transplantation of autologous microfractured adipose tissue both inside the fistulous tract and in peri-fistular tissues.
Figure 4: Graphical abstract of the MFAT harvesting and transplantation.
The adipose tissue performs an important regenerative and reparative function inside the fistular pathway, while in the peri-fistular tissues, in addition to a regenerative function, it also acts as a bulking agent that helps the progressive obliteration of the treated fistulous tracts.
Results
Our experience consists of three cases treated with a combination of VAAFT and microfractured adipose tissue grafts. All cases had undergone multiple interventions at the perianal level over a period ranging from 1 to 15 years. One case certainly failed due to poor patient compliance and personal habits that did not facilitate the healing process. This patient was then lost to follow-up. In the remaining two cases, the patients made a complete recovery with the disappearance of symptoms over a follow-up period of one to two years. The cases are briefly summarised in (Table 1).
Table 1: Clinical experience and results.
|
N |
Age |
G |
Initial diagnosis |
Previous treatment |
Interval from previous
treatment |
Symptoms |
MRI |
Treatments |
Results |
Notes |
|
Case 1 |
39 |
M |
High extra-sphincteric
perianal abscess, localized along left side of supra levator ani |
Since 2016 multiple surgical drainage and seton
position, supported by endoanal US at another hospital |
Several years up to 5y. 4 months from the last procedure in another hospital |
Pus discharge e severe pain |
“horse-shoe” anal fistula,
with internal orifice localized at midline posterior site of dentate line and
deep extension in ischio-anal space bilaterally |
VAAFT procedure and advancement mucosal flap to cover
internal orifice |
Recurrence |
|
|
VAAFT |
4 months |
Pus discharge |
2° VAAFT procedure and advancement mucosal flap to
cover internal orifice |
Recurrence |
|
|||||
|
VAAFT |
4 months |
Pus discharge from the internal orifice |
Combined approach of VAAFT plus
Lipogems® injection in the fistula tract and the ischio-anal space, harvesting
fat from abdomen |
No recurrence was observed at two-years follow-up. |
|
|||||
|
Case 2 |
48 |
M |
Complex perianal fistula with ascending pararectal and
trans sphincter pathway |
Fistulectoma and seton
positioning |
12 months |
Pus discharge e severe pain |
Large transphinteric
fistulous pathway with origin at 5 o'clock of the anus in the caudal course
and with cutaneous orifice at the left intergluteal level |
2° Fistulectoma and seton
positioning |
Recurrence |
Very low compliance |
|
Fistulectoma and seton positioning
|
5 months |
Pus discharge |
2° Fistulectoma and mucosal
flap |
Recurrence |
||||||
|
2° Fistulectoma and mucosal
flap |
8 months |
Severe pain |
Abscess dreinage |
Recurrence |
||||||
|
Abscess dreinage |
3 month |
Pus discharge |
VAAFT procedure and new mucosal flap to cover internal orifice |
Recurrence |
||||||
|
VAAFT procedure and new mucosal flap to cover internal orifice |
10 months |
Pus discharge |
LIFT and third mucosal flap |
Recurrence |
||||||
|
LIFT and third mucosal flap |
3 month |
Pus discharge |
Combined approach of VAAFT plus
Lipogems® injection harvesting fat from abdomen, mucosal flap |
Recurrence |
||||||
|
Combined approach of VAAFT plus Lipogems® injection
harvesting fat from abdomen, mucosal flap |
5 months |
Pus discharge |
Seton positioning |
Recurrence |
||||||
|
Seton positioning |
3 month |
Pus discharge |
VAAFT procedure and new mucosal flap to cover internal orifice +
Permacol placement |
Lost at follow up |
||||||
|
Case 3 |
54 |
M |
Relapsing anal abscesses with
double transphinteric and intersphincteric fistula up to the intergluteal
fold and with two cutaneous orifices |
Multiple abscess dreinage |
Several years up to 15y |
Pus discharge e severe pain |
Fistulous pathway with origin
in the anus at 6 o'clock and which goes beyond the internal anal sphincter
and divides into two branches, one with a transphinteric course that connects
to an abscess in the intergluteal area, with a cutaneous orifice and the
second with an inter-sphincter course up to the fold intergluetea with second
cutaneous orifice |
Seton positioning |
Recurrence |
|
|
Seton positioning |
4 months |
Pus discharge |
VAAFT procedure and new mio- mucosal flap to cover internal orifice
+ Permacol placement |
Recurrence |
|
|||||
|
VAAFT procedure and new mio- mucosal flap to cover internal orifice
+ Permacol placement |
6 month |
Pus discharge e severe pain |
Combined approach of VAAFT plus
Lipogems® injection harvesting fat from abdomen, mucosal flap |
No recurrence was observed at one year follow-up. |
|
Discussion
Anal fistulae represent one of the most challenging issues in proctology due to the involvement of anal sphincters and a significant recurrence rate observed in all surgical techniques [15]. In uncomplicated intersphincteric fistulas, classical fistulotomy has a 90% success rate with few post-operative complications, but in the case of medium or high transphincteric fistulas, classical fistulotomy presents a higher risk of faecal incontinence. New methods, such as anal plugs or fibrin glue, are associated with a low incidence of incontinence, but are burdened with a high rate of recurrence [16]. VAAFT is a mininvasive technique, and it is also sphincter-saving and feasible without complications in the case of multiple recurrences.
The VAAFT kit includes a fistuloscope (manufactured by Karl Storz SE & Co- Tuttlingen, Germany), an obturator, a unipolar electrode and an endo-brush, and is a procedure developed by Dr.med. Meinero in 2006. The VAAFT procedure normally requires a very short hospital stay, ranging from day hospital to a two-day post-operative hospital stay. A return to work is normally guaranteed within 10 days.
Human adult mesenchymal stem cells (MSCs) have been proposed as part of the treatment as they have been demonstrated to differentiate in-vitro into several cell lineages such as osteoblasts, chondrocytes, myocytes and adipocytes [17]. It has also been shown that human MSCs also can promote vasculogenesis, the main mechanism involved in tissue repair effectiveness by secreting a considerable number of bioactive molecules that have a paracrine action, sustaining angiogenetic, antifibrotic, antiapoptotic and immunomodulatory responses [18].
The therapeutic efficacy of MSCs is due to the so-called secretome, which is a set of proteins secreted into the extracellular space, including exosomes and non-coding RNAs [19]. Exosomes are small vesicles that penetrate the cell membrane and release biochemical messages that have been shown to control immune response, inhibit inflammation, promote angiogenesis and cell proliferation, and induce repair by means of tissue regeneration instead of a scarring process [20].
Adipose tissue offers the advantage of being easily accessible and having an abundance of MSCs. Until 2010 adipose-derived MSCs were obtained through GMP processing using enzymatic methods. The Lipogems® device, created in 2010 and clinically available since 2013, is an easy system for harvesting, processing and reinjecting the bioactive portion of adipose tissue in a one-step intervention without being subject to GMP processing and rules. After an easy harvest, the lipoaspirate is filtered, washed and exposed to slight mechanical forces without damaging the integrity of the stromal vascular niche and pericytes [21].
This system, characterised by minimal manipulation of the adipose tissue, enhances the natural regenerative properties of the receiving tissue and respects the natural healing process. It should also be noted that filtration and micro-fragmentation allow lipoaspirate particles with dimensions ranging between 0.3 and 0.8 mm to be obtained that can be easily re-injected using 20G cannulas or needles.
A thorough pre-operative study of the fistula through an endoanal ultrasound, computed tomography or, even better, MRI allows the surgical procedure to be better planned and accurately predicts which fistulous path should be explored and destroyed using VAAFT. The basic concept of VAAFT treatment is, indeed, the complete destruction of the fistular pathway.
Pre-operative pancoloscopy is equally useful as it is necessary for identifying any inflammatory bowel disease and in such cases the combination of pharmacological treatment is important. In patients with Crohn's disease, the incidence of perianal fistulas is reported to be between 13% and 39% and recent studies propose MSCs for the treatment of anal fistulas in subjects affected by this disease [22, 23]. The use of MSCs seems to be justified by the immunomodulatory power of mesenchymal cells, which express the surface antigens CD105, CD73 and CD90 [23, 24]. The action of MSCs appears to induce a local increase of regulatory T cells, thus mitigating the excessive immune response [25, 26].
Conclusion
In selected patients, the combination of video-assisted anal fistula treatment and injection of human autologous microfractured adipose tissue (MFAT) may be a valid, safe and feasible therapeutic option. Lipogems® injections have shown to be more effective in promoting tissue regeneration than “filling” the fistula tract [13].
The regenerative effect and the bulking agent function of adipose tissue are both probably facilitated after cleansing and debriding the fistula tract using the VAAFT system. It is worth noting that patients affected by recurrent fistulae should undergo a thorough pre-operative examination, usually through the performance of an endoanal UltraSound (US), tomography (inferior abdomen TC) and pelvic magnetic resonance (MR) in order to obtain a perfect morphological assessment, while colonoscopy and laboratory tests are fundamental for revealing inflammatory bowel diseases (Crohn or Ulcerative Colitis) [27, 28]. With regard to patients affected by inflammatory bowel diseases, surgical treatment needs to be supported by medical therapy, e.g., immunotherapy [29]. However, in these cases, treatment with microfractured autologous adipose tissue is indicated and recommended [24-26]. Although the study is innovative, due to its small sample size a randomized study ought to be performed with a larger sample size.
Funding
None.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
None.
Author Contributions
Marco De Monti: surgeon, clinical management of the case, writing and supervision of the paper, corrisponding Author. Giovanni Cestaro: study conception, acquisition of data, writer. Luca Regusci: proctologist, clinical management of the case. Fabrizio Fasolini: proctologist, clinical management of the case, former Chief of the Department. Ken Galetti: surgeon, proctologist, supervision of the paper, Chief of the Department.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 06, Sep 2022Accepted: Tue 20, Sep 2022
Published: Fri 07, Oct 2022
Copyright
© 2023 Marco De Monti. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2022.02.03
Author Info
Marco De Monti Giovanni Cestaro Luca Regusci Fabrizio Fasolini Ken Galetti
Corresponding Author
Marco De MontiEOC - Beata Vergine Regional Hospital, Department of Surgery, Mendrisio, Switzerland
Figures & Tables
Table 1: Clinical experience and results.
|
N |
Age |
G |
Initial diagnosis |
Previous treatment |
Interval from previous
treatment |
Symptoms |
MRI |
Treatments |
Results |
Notes |
|
Case 1 |
39 |
M |
High extra-sphincteric
perianal abscess, localized along left side of supra levator ani |
Since 2016 multiple surgical drainage and seton
position, supported by endoanal US at another hospital |
Several years up to 5y. 4 months from the last procedure in another hospital |
Pus discharge e severe pain |
“horse-shoe” anal fistula,
with internal orifice localized at midline posterior site of dentate line and
deep extension in ischio-anal space bilaterally |
VAAFT procedure and advancement mucosal flap to cover
internal orifice |
Recurrence |
|
|
VAAFT |
4 months |
Pus discharge |
2° VAAFT procedure and advancement mucosal flap to
cover internal orifice |
Recurrence |
|
|||||
|
VAAFT |
4 months |
Pus discharge from the internal orifice |
Combined approach of VAAFT plus
Lipogems® injection in the fistula tract and the ischio-anal space, harvesting
fat from abdomen |
No recurrence was observed at two-years follow-up. |
|
|||||
|
Case 2 |
48 |
M |
Complex perianal fistula with ascending pararectal and
trans sphincter pathway |
Fistulectoma and seton
positioning |
12 months |
Pus discharge e severe pain |
Large transphinteric
fistulous pathway with origin at 5 o'clock of the anus in the caudal course
and with cutaneous orifice at the left intergluteal level |
2° Fistulectoma and seton
positioning |
Recurrence |
Very low compliance |
|
Fistulectoma and seton positioning
|
5 months |
Pus discharge |
2° Fistulectoma and mucosal
flap |
Recurrence |
||||||
|
2° Fistulectoma and mucosal
flap |
8 months |
Severe pain |
Abscess dreinage |
Recurrence |
||||||
|
Abscess dreinage |
3 month |
Pus discharge |
VAAFT procedure and new mucosal flap to cover internal orifice |
Recurrence |
||||||
|
VAAFT procedure and new mucosal flap to cover internal orifice |
10 months |
Pus discharge |
LIFT and third mucosal flap |
Recurrence |
||||||
|
LIFT and third mucosal flap |
3 month |
Pus discharge |
Combined approach of VAAFT plus
Lipogems® injection harvesting fat from abdomen, mucosal flap |
Recurrence |
||||||
|
Combined approach of VAAFT plus Lipogems® injection
harvesting fat from abdomen, mucosal flap |
5 months |
Pus discharge |
Seton positioning |
Recurrence |
||||||
|
Seton positioning |
3 month |
Pus discharge |
VAAFT procedure and new mucosal flap to cover internal orifice +
Permacol placement |
Lost at follow up |
||||||
|
Case 3 |
54 |
M |
Relapsing anal abscesses with
double transphinteric and intersphincteric fistula up to the intergluteal
fold and with two cutaneous orifices |
Multiple abscess dreinage |
Several years up to 15y |
Pus discharge e severe pain |
Fistulous pathway with origin
in the anus at 6 o'clock and which goes beyond the internal anal sphincter
and divides into two branches, one with a transphinteric course that connects
to an abscess in the intergluteal area, with a cutaneous orifice and the
second with an inter-sphincter course up to the fold intergluetea with second
cutaneous orifice |
Seton positioning |
Recurrence |
|
|
Seton positioning |
4 months |
Pus discharge |
VAAFT procedure and new mio- mucosal flap to cover internal orifice
+ Permacol placement |
Recurrence |
|
|||||
|
VAAFT procedure and new mio- mucosal flap to cover internal orifice
+ Permacol placement |
6 month |
Pus discharge e severe pain |
Combined approach of VAAFT plus
Lipogems® injection harvesting fat from abdomen, mucosal flap |
No recurrence was observed at one year follow-up. |
|

1: Spike for wash solution bag; 2: Soft drip chamber; 3: Washing line inlet clamp; 4: Washing line; 5: Input clamp; 6: Lipoaspirate loading valve; 7: Blue head (inlet filter); 8: Processing unit; 9: Gray head (outlet filter); 10: MFAT extraction valve; 11: Clamp discharge line; 12: Discharge line; 13: Discharge bag connection clamp; 14: Discharge bag connection; 15: Discharge bag clamp; 16: Discharge bag.
References
1.
Newman PA, Dixon T
(2017) Benign anorectal conditions. Surgery 35: 443-450.
2.
Whiteford MH (2007)
Perianal Abscess/Fistula Disease. Clin Colon
Rectal Surg 20: 102-109. [Crossref]
3.
Meinero P, Mori L
(2011) Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving
procedure for treating complex anal fistulas. Tech Coloproctol 15:
417-422. [Crossref]
4.
Ferrario L, Cestaro
G, Meinero P, Fasolini F, De Monti M (2020) Video Assisted Anal Fistula
Treatment (VAAFT) for Treating a Complex Posttraumatic Anal Fistula: Case
Report. Am J Surg Case Report 2: 1-3.
5.
Meinero P, Mori L,
Gasloli G (2014) Video-assisted anal fistula treatment: A new concept of
treating anal fistulas. Dis Colon Rectum 57: 354-359. [Crossref]
6.
Giamundo P, Geraci
M, Tibaldi L, Valente M (2014) Closure of fistula‐in‐ano with laser-FiLaC™: an
effective novel sphincter‐saving procedure
for complex disease. Colorectal Dis 16: 110-115. [Crossref]
7.
Rojanasakul A
(2009) LIFT procedure: a simplified technique for fistula-in-ano. Tech
Coloproctol 13: 237-240. [Crossref]
8.
Cestaro G, Gentile
M (2020) Anal fistulas treatment with bulking agents: an observational study. Chirurgia
33: 130-133.
9.
Giordano P, Sileri
P, Buntzen S, Nunoo Mensah J, Lenisa L et al. (2018) Final results of a
European, multicentre, prospective, observational study of Permacol™ collagen
paste injection for the treatment of anal fistula. Colorectal Dis 20:
243-251. [Crossref]
10.
Tremolada C,
Ventura C, Gorio A, Carelli S, Ricordi C et al. (2013) A new device (Lipogems®)
to obtain micronized fat tissue with a highly preserved SVF for
autologous/allogenic use in Regenerative Medicine: clinical use and three
years’ experience. In International Society of Plastic Regenerative Surgery
(ISPRES).
11.
Jevotovsky DS,
Alfonso AR, Einhorn TA, Chiu ES (2018) Osteoarthritis and stem cell therapy in
humans: a systematic review. Osteoarthritis Cartilage 26: 711-729. [Crossref]
12.
Klar AS, Zimoch J,
Biedermann T (2017) Skin tissue engineering: application of adipose-derived
stem cells. BioMed Res Int 2017: 9747010. [Crossref]
13.
Giori A, Tremolada C,
Vailati R, Navone SE, Marfia G et al. (2015) Recovery of function in anal incontinence after micro-fragmented fat
graft (Lipogems®) injection: two years follow up of the first 5 cases. CellR4
3: e1544.
14.
Topal U, Eray İC,
Rencüzoğulları A, Yalav O, Alabaz Ö (2019) Short-term results of
adipose-derived stem cell therapy for the treatment of complex perianal
fistula. Ann Ital Chir 90: 583-589. [Crossref]
15.
Mei Z, Wang Q,
Zhang Y, Liu P, Ge M et al. (2019) Risk factors for recurrence after anal
fistula surgery: A meta-analysis. Int J Surg 69: 153-164. [Crossref]
16.
Garg P, Singh P
(2017) Video-Assisted Anal Fistula Treatment (VAAFT) in Cryptoglandular
fistula-in-ano: A systematic review and proportional meta-analysis. Int J
Surg 46: 85-91. [Crossref]
17.
Caplan AI (2007)
Adult mesenchymal stem cells for tissue engineering versus regenerative
medicine. J Cell Physiol 213: 341-347 . [Crossref]
18.
Ventura C, Cantoni
S, Bianchi F, Lionetti V, Cavallini C et al. (2007) Hyaluronan mixed esters of
butyric and retinoic acid drive cardiac and endotelial fate in term placenta
human mesenchymal stem cells and enhance cardiac repair in infarcite rat
hearts. J Biol Chem 282: 14243-14252. [Crossref]
19.
NajarM, Melki R,
Khalife F, Lagneaux L, Bouhtit F et al. (2022) Therapeutic Mesenchymal Stem/Stromal
Cells: Value, Challenges and Optimization. Front Cell Dev Biol 9:
716853. [Crossref]
20.
Vu NB, Nguyen HT,
Palumbo R, Pellicano R, Fagoone S et al. (2021) Stem cell-derived exosomes for
wound healing: current status and promising directions. Minerva Med 112:
384-400. [Crossref]
21.
Bianchi F, Maioli
M, Leonardi E, Olivi E, Pasquinelli G et al. (2013) A new non enzymatic method and device to obtain a fat tissue derivative
highly enriched in pericyte-like elements by mild me- chanical forces from
human lipoaspirates. Cell Transplant 22: 2063-2077. [Crossref]
22.
Naldini G, Sturiale
A, Fabiani B, Giani I, Menconi C (2018) Micro‐fragmented adipose tissue
injection for the treatment of complex anal fistula: a pilot study accessing
safety and feasibility. Tech Coloproctol 22: 107-113. [Crossref]
23.
Gallo G, Tiesi V,
Fulginiti S, De Paola G, Vescio G et al. (2020) Mesenchymal Stromal Cell Therapy in the
Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review. Medicina 56: 563. [Crossref]
24.
Martínez Montiel
Mdel P, Gómez Gómez GJ, Flores AI (2014) Therapy with stem cells in
inflammatory bowel disease. World J Gastroenterol 20: 1211-1227. [Crossref]
25.
Wang HS, Hung SC,
Peng ST, Huang CC, Wei HM et al. (2004) Mesenchymal stem cells in the Wharton’s
jelly of the human umbilical cord. Stem Cells 22: 1330-1337. [Crossref]
26. English K (2013) Mechanisms of mesenchymal stromal cell immunomodulation. Immunol
Cell Biol 91: 19-26. [Crossref]
27.
Amato A,
Bottini C, De Nardi P, Giamundo P, Lauretta A et al. (2020) Evaluation and
management of perianal abscess and anal fistula: SICCR position statement. Tech
Coloproctol 24: 127-143. [Crossref]
28. Flynn S, Eisenstein S (2019) Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am 99: 1051-1062. [Crossref]
29. Bai A, Peng Z (2010) Biological therapies of inflammatory bowel disease. Immunotherapy 2: 727-742. [Crossref]
