Relevance of Preoperative Vessel Mapping and Early Postoperative Ultrasonography in Predicting AV Fistula Failure in Chronic Kidney Disease Patients
A B S T R A C T
Introduction: The increasing prevalence of chronic kidney disease (CKD), coupled with advancements in the diagnosis and treatment of renal diseases and improvements in life expectancy, has led to a greater number of patients requiring hemodialysis. The preferred method of vascular access for hemodialysis is AV fistula formation; however, it is associated with a high rate of failure. In our prospective study, we focused on 40 CKD patients planned for initiation of maintenance hemodialysis.
Methods: We employed preoperative ultrasound mapping to assess cephalic vein diameter, compressibility, and colour flow, as well as radial and brachial artery diameter, peak systolic velocity, and intimal wall calcification. Postoperatively, ultrasound examinations were conducted on day 7 and at 6 weeks to evaluate fistula blood volume and detect any complications.
Results: A significant association between fistula failure and cephalic vein diameter, brachial artery diameter, intimal vessel wall calcification, and comorbid conditions like diabetes mellitus was observed. Furthermore, blood flow at day 7 was notably lower in the failure group compared to those with a functioning fistula and any fistula with blood flow <154 ml/min on day 7 may be predictive of early fistula failure.
Conclusion: Preoperative vessel mapping and early postoperative ultrasonography is indispensable for patients who require AV fistula formation for hemodialysis and provide valuable information for selecting suitable vessels for successful fistula creation and enable early intervention to salvage a failing fistula after the surgery. By utilizing these, healthcare professionals can make informed decisions and take necessary steps to optimize the outcomes of AV fistula formation in patients undergoing hemodialysis.
Keywords
Av fistula, venous mapping, hemodialysis, vessel mapping
Introduction
Chronic kidney disease(CKD) is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health (KDIGO 2012). Number of patients who need hemodialysis in CKD has increased due to advances in treatment of renal diseases and overall increase in life expectancy. Hemodialysis is the renal replacement modality in 70% patients with end stage renal disease and renal failure (GFR as per KDIGO CKD classification <15 ml/min/1.73 metre square) [1].
The preferred type of dialysis access is arteriovenous fistula, but when it fails, it can cause health problems and lead to hospitalization in hemodialysis patients [1]. Having clear criteria using sonography to identify fistulas that may fail in the early postoperative period would be invaluable for patient care [2]. Conducting a comprehensive preoperative assessment through colour doppler ultrasound and mapping of the arterial and venous vascular system can increase the proportion of patients suitable for AVF placement and improve the cumulative patency rate of fistulas [3, 4].
Blood flow in an AV fistula experiences a rapid increase following creation, peaking between 4 to 12 weeks, with 40% to 60% of the overall increase occurring within 24 hours of the procedure [2]. Taking these factors into account, measuring blood flow through doppler ultrasonography during the early postoperative period (day 7) in an AV fistula could potentially predict its failure to mature.
Methods
The study was conducted at the at department of radiodiagnosis of a tertiary care medical college and hospital in western Uttar Pradesh, India using Samsung V8 ultrasound machine with linear probe of high frequency range 4-18 Mhz for vascular assessment. After receiving approval from the local ethical committee and obtaining written consent from all participating patients, the study was carried out from May 2022 to April 2023. A total of 40 patients diagnosed with CKD grade 5 requiring dialysis based on clinical impressions, as well as imaging and blood investigations, referred by the departments of nephrology and cardiothoracic and vascular surgery (CTVS) for scheduled fistula formation were included in the study (GFR as per KDIGO CKD classification <15 ml/min/1.73 metre square body surface area).
The study included patients diagnosed as CKD grade 5 and planned for their first time hemodialysis AVF, as well as patients with a malfunctioning AVF requiring the creation of a new fistula in another limb. Patients with previous fistula formation in the same upper limb, poor vascularity (such as evident vessel wall calcification and very small caliber vessels), and those with a newly created brachiobasilic fistula were excluded from the study.
Preoperative vascular mapping involving a comprehensive examination of both arterial and venous structures. For arterial assessment, the radial artery at the wrist and the brachial artery at the antecubital fossa are examined for diameter adequacy, with a diameter greater than 0.20 cm for radial artery and 0.35 cm for brachial artery considered suitable. The presence of intimal calcification, peak systolic velocity, and anatomical variations such as high brachial artery bifurcation are evaluated. To confirm the patency of the deep palmar arch, the modified duplex allen test is performed.
On the venous side, the cephalic vein, often used in forearm arteriovenous fistulas (AVFs), is assessed for compressibility to look for thrombus, and size, typically measuring around 0.25 cm in minimal diameter. Depth from skin is assessed as superficial veins are preferred. Site of venous stenosis and sclerotic/thick-walled veins are noted. The entire vein is assessed upto insertion in subclavian vein. Any large branches from the vein are noted, as their presence can impact maturation. In case cephalic vein in arm was small/ thrombosed, it drains via large antecubital vein into basilic / brachial vein , in such case forearm fistula was still possible as long as diameter thresholds are maintained. Bilateral examination of the internal jugular vein (IJV) and subclavian vein is carried out to rule out outflow stenosis based on respiratory phasicity and transmitted cardiac pulsations.
I Technique
A comprehensive clinical history and laboratory tests were recorded for all patients. During the examination, the patient's arm was positioned comfortably, abducted at around a 45° angle from the body. Vascular assessment was conducted using both transverse and longitudinal planes, employing gentle compression and coupling gel on the probe to optimize imaging quality. To minimize the risk of infection or bleeding from the puncture site, postoperative doppler ultrasonography examination of the dialysis fistula was conducted prior to dialysis sessions. Blood flow measurements of the fistula were taken without applying compression. The evaluation schedule including preoperative assessment, a follow-up at 7 days postoperatively, and another evaluation at 6 weeks ("Rule of 6" for predicting AVF maturity) (Figure 1).
Figure 1: Arterialised cephalic vein showing diameter ~ 6.53 mm and depth from skin 1.5 mm (< 6 mm) (Appropriate according to KDOQI guidelines ‘Rule of 6’).
II Statistical Analysis
Data collected for the study were subjected to statistical analysis using SPSS version 20. Descriptive statistics such as mean (X) and standard deviation (SD) were utilized to describe quantitative data, including age, BMI, cephalic vein diameter, radial artery diameter, brachial artery diameter, day 7 postoperative blood flow, and 6-week postoperative blood flow. Qualitative data, such as mature and failed fistula, causes of fistula failure, gender, and diabetes mellitus, were presented as numbers and percentages. Analytical statistics, including the chi-squared test, Fischer's exact test, Student's t-test were applied to determine associations between factors and the targeted disease. ROC curves were used to evaluate the sensitivity and specificity of fistula blood flow measured in early postoperative periods for detecting AV fistula failure. A p-value of >0.05 was considered statistically non-significant, while a p-value of <0.05 was considered statistically significant.
Observations and Results
This study involved 40 patients who needed a new upper limb fistula. The participants were evenly divided between those below 50 years old and those aged 50 or above, as well as between males and females. Nearly half of the patients had no co-morbidities, while 30% had diabetes mellitus. A subset of patients had a body mass index above 25 kg/m², and 12.5% had hypertension/coronary artery disease. Measurements revealed that forearm cephalic vein had a mean diameter of 2.21 ± 0.5 mm, while the arm cephalic vein had a mean diameter of 3.04 ± 0.79 mm. All patients displayed vein compressibility. The radial artery had a mean diameter of 2.1 ± 0.37 mm, and the brachial artery had a mean diameter of 3.61 ± 0.7 mm. The peak systolic velocity was approximately 51.4 ± 16.1 cm/sec. A significant portion of the patients (45%) had arterial intimal wall calcification.
During postoperative period, the mean fistula blood flow was 309.75 ± 207.64 mL/min on day 7 and 499.04 ± 353.22 mL/min at 6 weeks. Most patients (60%) did not experience any complications (Figure 2), while the rest (16 out of 40) had thrombosis (22.50%) (Figure 3), stenosis(7.50%), failed maturation (10%) (Figure 4), or hematoma (5%) (Figure 5). Distribution of AVF failure was comparable with age and gender (Table 1). AVF failure was significantly higher in patients with co-morbidities as compared to without co-morbidities. AVF failure was significantly higher in diabetics as compared to non-diabetics. Distribution of AVF failure was comparable with hypertension/coronary artery disease and body mass index>25 kg/m². (Table 1).
Table
1: Association
of age (years), gender and comorbidities with AVF failure.
|
Gender |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Female |
10 (50%) |
10 (50%) |
20 (100%) |
0.197† |
|
Male |
6
(30%) |
14
(70%) |
20
(100%) |
|
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
Age(years) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
<50 |
5 (25%) |
15 (75%) |
20 (100%) |
0.053† |
|
>=50 |
11
(55%) |
9
(45%) |
20
(100%) |
|
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
Co-morbidity |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
No co-morbidity |
||||
|
No |
12
(57.14%) |
9
(42.86%) |
21
(100%) |
0.027* |
|
Yes |
4 (21.05%) |
15 (78.95%) |
19 (100%) |
|
|
Diabetes
mellitus |
||||
|
No |
7 (25%) |
21 (75%) |
28 (100%) |
0.005* |
|
Yes |
9
(75%) |
3
(25%) |
12
(100%) |
|
|
Hypertension/Coronary artery disease |
||||
|
No |
14
(40%) |
21
(60%) |
35
(100%) |
1* |
|
Yes |
2 (40%) |
3 (60%) |
5 (100%) |
|
|
Body
mass index>25 kg/m² |
||||
|
No |
14 (41.18%) |
20 (58.82%) |
34 (100%) |
1* |
|
Yes |
2
(33.33%) |
4
(66.67%) |
6
(100%) |
|
† Chi square test.
*
Fisher's exact test.
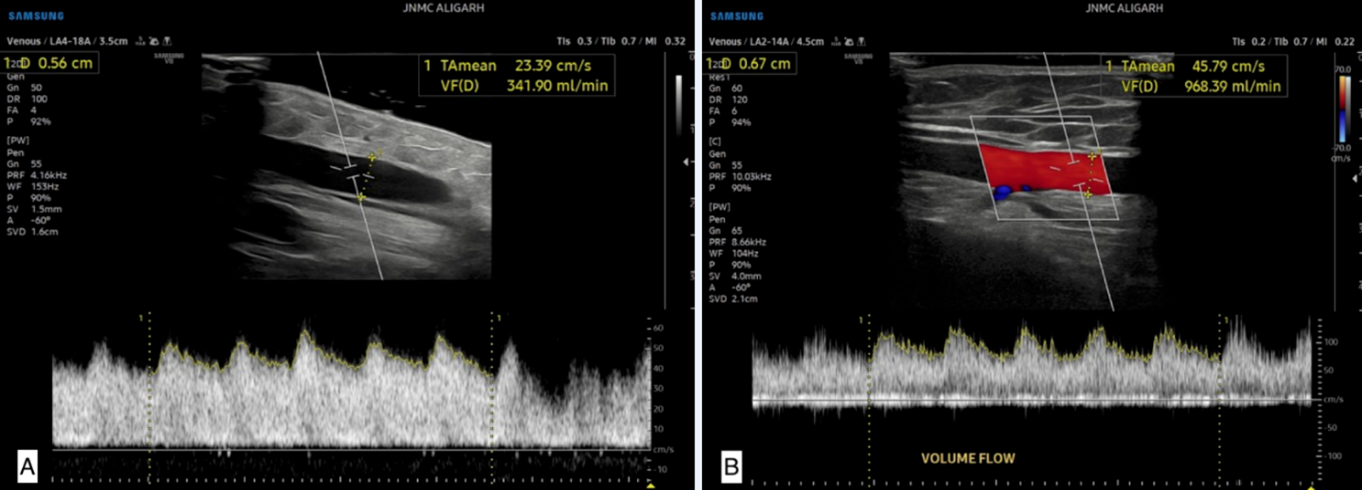
Figure 2: A 45-year-old male patient with history of chronic kidney disease. A) Doppler US 7 days after the surgery shows the blood flow 10 cm cranial to the anastomosis (volume flow is 341.90 ml/min). B) Color Doppler US shows the late postoperative US findings 10 cm cranial to the anastomosis (volume flow is 968.39 ml/min). The AV fistula matured showing adequate flow.
Figure 3: A 62 y female patient with history of diabetes mellitus. A) Doppler US 7 days after the surgery shows the blood flow 10 cm away from the distal radiocephalic fistula (volume flow measurement is 117.5 ml/min). B) USG at 6 weeks demonstrate total thrombosis of the cephalic vein, and no color flow was detected. C) This fistula failed, and the cause of failure was complete thrombosis of the cephalic vein.
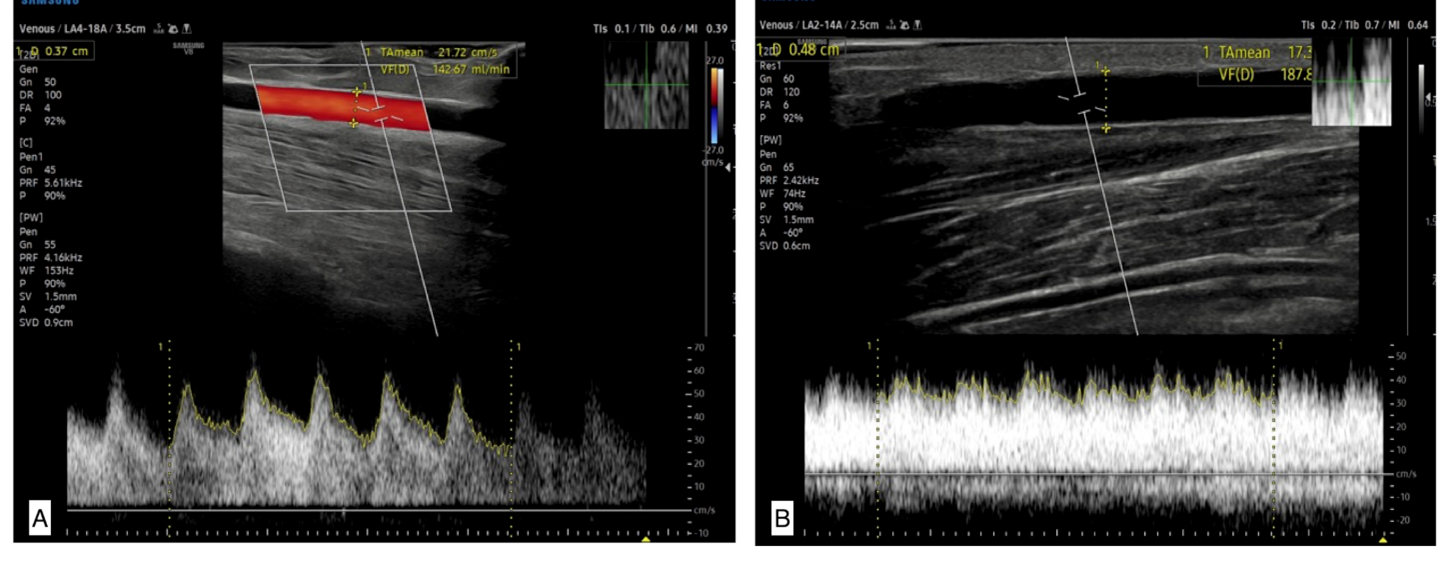
Figure 4: A 32-year-old female patient with history of chronic kidney disease. A) Color Doppler USG 7 days after the fistula formation shows volume flow of 142.6 ml/min). B) Doppler USG during late postoperative period demonstrate volume flow measurement of 187 ml/ min, the fistula could not support adequate blood flow and failed to mature.
Figure 5: A 54-year-old male patient with history of diabetic nephropathy. A) Color Doppler and B mode USG demonstrates hematoma related to the AV fistula at the day 7 of postoperative period, and it measures (2. × 1.9 cm). B) Patient later developed thrombosis in cephalic vein leading to fistula failure.
Significant association was seen in cephalic venous diameter of forearm and arm with AVF failure. Mean cephalic venous diameter of forearm and arm in patients without AVF failure was significantly higher as compared to patients with AVF failure. Mean of depth from skin in patients with AVF failure was higher than in patients without AVF failure with no significant association between them (Table 2).
Table
2: Association
of cephalic venous diameter(mm) and its depth from skin(mm) with AVF failure.
|
Cephalic
venous diameter(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Forearm |
||||
|
Mean
± SD |
2.01
± 0.5 |
2.35
± 0.49 |
2.21
± 0.52 |
0.037‡ |
|
Median(25th-75th percentile) |
1.95(1.7-2.2) |
2.3(2.175-2.525) |
2.2(1.9-2.5) |
|
|
Range |
1.1-3.1 |
1.1-3.6 |
1.1-3.6 |
|
|
Arm |
||||
|
Mean
± SD |
2.51
± 0.54 |
3.4
± 0.73 |
3.04
± 0.79 |
0.0002‡ |
|
Median(25th-75th percentile) |
2.45(2.175-2.725) |
3.3(2.875-3.6) |
2.9(2.575-3.4) |
|
|
Range |
1.7-3.9 |
2.5-5.5 |
1.7-5.5 |
|
|
Depth
from skin(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Mean ± SD |
4.19 ± 1.87 |
3.34 ± 1.32 |
3.68 ± 1.6 |
0.101‡ |
|
Median(25th-75th
percentile) |
4.35(2.75-5.175) |
3.1(2.375-4.625) |
3.3(2.375-4.925) |
|
|
Range |
1.5-8.5 |
1.5-5.5 |
1.5-8.5 |
‡
Independent t test.
No significant association was seen in radial artery diameter with AVF failure. Mean of radial artery (mm) in patients with AVF failure was 1.97 ± 0.29 and in patients without AVF failure was 2.19 ± 0.39 with no significant association between them. Significant association was seen in brachial artery diameter in patients with AVF failure. Mean of brachial artery diameter in patients without AVF failure was significantly higher as compared to patients with AVF failure (Table 3). Mean of PSV (cm/sec) in patients with AVF failure and without AVF failure showed no significant association between them (Table 3). AVF failure was significantly higher in patients with intimal wall calcification in artery as compared to patients without intimal wall calcification (Table 3). Significant association was seen in fistula blood flow (mL/min) on day 7 and at 6 weeks with AVF failure. Mean fistula blood flow (mL/min) on day 7 and at 6 weeks in patients without AVF failure was significantly higher as compared to patients with AVF failure. (Table 4). The cutoff value for fistula failure in early postoperative period (day 7) was 154 ml/min (Table 5).
Table
3: Association
of artery diameter(mm) , PSV(cm/sec) and intimal wall calcification with AVF
failure.
|
Artery
diameter(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Radial artery |
||||
|
Mean
± SD |
1.97
± 0.29 |
2.19
± 0.39 |
2.1
± 0.37 |
0.064‡ |
|
Median(25th-75th percentile) |
2(1.8-2.1) |
2.15(1.9-2.325) |
2.1(1.9-2.2) |
|
|
Range |
1.2-2.5 |
1.5-3.1 |
1.2-3.1 |
|
|
Brachial artery |
||||
|
Mean
± SD |
3.34
± 0.6 |
3.79
± 0.72 |
3.61
± 0.7 |
0.046‡ |
|
Median(25th-75th percentile) |
3.15(2.8-3.925) |
3.6(3.475-3.85) |
3.6(3.1-3.925) |
|
|
Range |
2.6-4.4 |
2.9-5.4 |
2.6-5.4 |
|
|
PSV(cm/sec) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|||||
|
Mean ± SD |
55.93 ± 17.04 |
48.38 ± 15.1 |
51.4 ± 16.13 |
0.149‡ |
|||||
|
Median(25th-75th
percentile) |
54 |
45.5 |
51 |
||||||
|
Range |
33-100 |
29-75 |
29-100 |
||||||
|
Intimal
wall calcification in artery |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
||||
|
No |
3 (13.64%) |
19 (86.36%) |
22 (100%) |
0.0003* |
|
||||
|
Yes |
13
(72.22%) |
5
(27.78%) |
18
(100%) |
|
|||||
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
|||||
‡ Independent t test.
*
Fisher's exact test.
Table
4: Association
of fistula blood flow(mL/min) with AVF failure.
|
Fistula
blood flow(mL/min) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
On day 7 |
||||
|
Mean
± SD |
91.62
± 41.6 |
455.17
± 129.68 |
309.75
± 207.64 |
<.0001‡ |
|
Median(25th-75th percentile) |
82.5(63.75-127.75) |
445(402.25-556.25) |
391(89.25-451.5) |
|
|
Range |
10-154 |
78-670 |
10-670 |
|
|
At 6 weeks |
||||
|
Mean
± SD |
145.41
± 77.76 |
734.79
± 250.25 |
499.04
± 353.22 |
<.0001‡ |
|
Median(25th-75th percentile) |
135.75(100-200.5) |
690(653-768.5) |
608(157-703) |
|
|
Range |
10-290 |
154-1500 |
10-1500 |
|
‡
Independent t test.
Table
5: Receiver
operating characteristic curve of Fistula blood flow(mL/min) on day 7 for
predicting AVF failure.
|
Variables |
Fistula
blood flow(mL/min) on day 7 |
|
Area under the ROC curve (AUC) |
0.978 |
|
Standard
Error |
0.0227 |
|
95% Confidence interval |
0.873 to 1.000 |
|
P
value |
<0.0001 |
|
Cut off |
≤154 |
|
Sensitivity(95%
CI) |
100%(79.4
- 100.0%) |
|
Specificity(95% CI) |
95.83%(78.9 - 99.9%) |
|
PPV(95%
CI) |
94.1%(71.3
- 99.9%) |
|
NPV(95% CI) |
100%(85.2 - 100.0%) |
|
Diagnostic
accuracy |
97.50% |
Discussion
Chronic kidney disease (CKD) refers to structural or functional abnormalities in the kidneys that persist for more than three months and have implications for one's health (KDIGO 2012). The stages of CKD are determined based on the level of kidney function, with kidney failure being defined as a glomerular filtration rate (GFR) below 15 ml/min/1.73m². The decision to initiate hemodialysis in patients with CKD is typically made through collaboration between the nephrologist and the patient. Dialysis offers several advantages, including the potential improvement of uremic symptoms and volume overload that have not responded to medical management. Additionally, it can help lower the risk of life-threatening complications associated with kidney failure [6].
The preferred vascular access type for hemodialysis is an arteriovenous fistula. However, fistula failure is a common issue in these patients, and early confirmation of failure can be clinically challenging. Therefore, it is crucial to assess the fistula early using ultrasonography and establish specific criteria based on ultrasound findings to predict potential failure. Prior to the procedure, obtaining a clinical history that includes comorbidities and conducting vessel mapping using ultrasound to identify any abnormalities in the access vessels that could contribute to fistula failure during or after surgery, thereby helping to minimize unnecessary incisions and optimize the success of the procedure [2].
Our research revealed a significant correlation between the diameter of the cephalic vein preoperatively and the occurrence of arteriovenous fistula (AVF) failure. We observed that the mean cephalic vein diameter in patients without AVF failure was higher compared to those who experienced AVF failure. These findings indicate that the cephalic vein diameter may play a crucial role in the development of AVF failure. A larger diameter is potentially associated with a lower risk of AVF failure, allowing better blood flow for hemodialysis. Studies in the past have also supported these findings. Wong reported that all AVF failed if the venous diameter at the wrist was 1.6 mm or less [7]. Mendes demonstrated successful maturation in patients when the cephalic vein diameter exceeded 2 mm [8]. Silva proposed a minimum vein diameter of 2.5 mm for adequate maturation [9]. There was no significant difference observed in the depth of the vein from the skin between functional and failed fistulas in our study. Despite this, surgeons generally prefer veins that are more superficial because they are easier to access.
We found that the mean preoperative diameter of the radial and brachial arteries in the failure group was lower compared to patients without fistula failure. However, a significant association was only observed in the brachial artery diameter. Previous studies have presented varying perspectives on this matter. Kakkos concluded that a brachial artery diameter of 4.1 mm or less in brachiocephalic arteriovenous fistulas was associated with lower functionality [10]. Misskey discovered that a combination of a radial artery diameter of less than 2.1 mm and a venous diameter of less than 3.0 mm provided the best prediction of failure [11]. Ren noted that incidence of complications decreased as the brachial artery diameter increased [12].
We found a significant association between arterial intimal wall calcification and rates of arteriovenous fistula (AVF) failure compared to patients without intimal wall calcification. Vascular calcification contributes to increased arterial stiffness, leading to excessive shear stress, turbulence, and neointimal hyperplasia [13]. These ultimately result in the failure of arterial remodeling after AVF creation. Supporting our findings, a 5-year follow-up study conducted by Jankovic demonstrated that the presence and severity of arterial calcification were significant predictors of AVF failure and could guide the selection of the suitable vascular access for hemodialysis patients [14]. However, Allon involved 127 CKD patients undergoing AVF surgery and showed that arterial microcalcification, did not explain postoperative AVF stenosis, AVF non-maturation, or AVF failure after successful cannulation [15].
Among the 16 patients who experienced AVF failure, 11 were aged 50 years or older, and 10 out of the 16 patients with AVF failure were female. However, it is important to note that these findings did not reach statistical significance in our analysis. Nonetheless, Yap suggested that older age and female gender are more prevalent in the group of patients experiencing early AVF failure, possibly due to higher vascular stiffness and smaller vessel diameters in females [16]. Venkatnarayana found that female gender, particularly in individuals aged 65 years or older, had a higher risk of poor unassisted AVF patency [17].
Findings regarding the risk factors for AV fistula failure align with meta-analysis conducted by Yan, which examined the association between diabetes and AVF failure in dialysis patients and demonstrated a statistically significant higher rate of AVF failure in diabetic patients, highlighting diabetes mellitus as a risk factor for failure [18]. Our study also found diabetes mellitus as a significant cause of end-stage renal disease, consistent with previous studies [19]. Study by Ahmadi involving 400 patients reported that diabetes did not increase the risk of early AVF failure, while patients with a previous history of hypertension had a lower risk of AVF failure [20]. Gordon concluded that diabetic patients should not be discouraged from undergoing AVF formation, as they have comparable outcomes to non-diabetic patients, with AVF remaining the optimal access for dialysis [21].
We did not find a significant association between AVF failure and a body mass index (BMI) greater than 25 kg/m². Similarly, Kats reported that obesity is only linked to secondary AVF failure in individuals with a BMI of 30 kg/m² or higher, rather than an increased risk of primary failure [22]. However, it is worth noting that obesity can present technical challenges during AVF creation due to the depth of the involved vessels. Therefore, it becomes important to conduct a thorough assessment of hemodynamic factors through vascular mapping in obese patients before proceeding with AVF creation. In our study, the mean preoperative arterial peak systolic velocity (PSV) was determined to be 51.4 cm/sec with no statistically significant difference between failure and mature group. Previous research conducted by Sedlacek, suggested that a PSV of at least 50 cm/sec was necessary for a successful fistula [23]. Lockhart excluded arteries measuring less than 2 mm in diameter and found no difference in preoperative PSV between fistulae that were deemed adequate or inadequate [24].
We observed a significant association between fistula blood flow on day 7 and at 6 weeks with AVF failure. Patients without failure had a significantly higher mean blood flow on day 7 and at 6 weeks compared to those with failure. A blood flow cutoff value of 154 ml/min on day 7 was determined for distinguishing functioning and failing fistulas. Robbin, reported that the blood flow rate in AVF at 1 day is typically over 50% of the blood flow rate at 6 weeks and measurements taken at the two-week mark were reliable indicators of the diameter and blood flow at 6 weeks, allowing for early identification of fistulas that are unlikely to develop optimally [25]. In study by Ladenheim, none of the fistulas with a blood flow below 200 mL/minute at 1-week postoperative visit reached maturity without undergoing a maturation procedure [26]. Shintaku identified a flow volume of 235 mL/min and a resistive index of 0.63 on day 1 as thresholds for predicting early fistula failure [27].
During the follow-up period of our study, 40% of arteriovenous fistulas (16 out of 40) experienced failure. Failed fistulas were attributed to various factors, including thrombosis, failure to mature, stenosis, and hematoma. Primary failure occurs when an access is unable to provide adequate blood flow for dialysis even after a reasonable period of maturation. This can happen when the draining vein fails to adequately dilate or when the feeding artery does not supply sufficient blood flow [19]. In study by Venkat Ramanan, primary failure was observed in 90 out of 352 cases (25.6%) [28]. Bylsma reported a primary patency rate of 64% at 1 year [29]. Schinstock noted a primary failure rate of 37.1% [30].
The high failure rate observed in our study could be attributed to the fact that our study was conducted in a teaching hospital where the surgical team consisted of surgeons with varying levels of experience in performing AVF surgeries. Future studies focusing on vascular access outcomes should consider incorporating the extent of surgeon training and experience in the analysis. Additionally, the lack of sufficient patient education regarding proper self-care practices for fistulas could have contributed to the observed failure rate. It is crucial to ensure that patients receive education and guidance on proper care of their fistulas.
It is important to acknowledge the limitations of our study. Firstly, the study included a small number of patients from a single center, which restricts the generalizability of our findings to other healthcare settings. Additionally, some cases had to be excluded due to the need for more extended follow-up. Future studies with larger sample sizes and multi-center participation, may provide more comprehensive and generalizable results.
Conclusion
Integration of a thorough clinical history and ultrasonography can establish standardized criteria for predicting AV fistula failure. Preoperative ultrasonography, which includes vessel mapping can serve as a valuable guidance for successful fistula creation. Furthermore, early postoperative ultrasonography, specifically performed on day 7, proves to be a useful tool in predicting fistula failure and blood flow measurement of less than 154 ml/min at day 7 may indicate a higher risk of failure. Our study emphasizes the importance of preoperative venous mapping in all patients posted for AVF surgery as the standard of care , however due to lack of awareness amongst operating team and patient inertia, significant proportion of patients develop primary failure which can be avoided in most circumstances.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 18, Dec 2023Accepted: Sat 06, Jan 2024
Published: Thu 08, Feb 2024
Copyright
© 2023 Saif Quaiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2023.02.02
Author Info
Shikhar Gupta Mehtab Ahmed Sayema . Azam Haseen Saif Quaiser
Corresponding Author
Saif QuaiserAssistant Professor, Nephrology Unit, Department of Medicine, Jawaharlal Nehru Medical College, Aligarh, Uttar Pradesh, India
Figures & Tables
Table
1: Association
of age (years), gender and comorbidities with AVF failure.
|
Gender |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Female |
10 (50%) |
10 (50%) |
20 (100%) |
0.197† |
|
Male |
6
(30%) |
14
(70%) |
20
(100%) |
|
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
Age(years) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
<50 |
5 (25%) |
15 (75%) |
20 (100%) |
0.053† |
|
>=50 |
11
(55%) |
9
(45%) |
20
(100%) |
|
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
Co-morbidity |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
No co-morbidity |
||||
|
No |
12
(57.14%) |
9
(42.86%) |
21
(100%) |
0.027* |
|
Yes |
4 (21.05%) |
15 (78.95%) |
19 (100%) |
|
|
Diabetes
mellitus |
||||
|
No |
7 (25%) |
21 (75%) |
28 (100%) |
0.005* |
|
Yes |
9
(75%) |
3
(25%) |
12
(100%) |
|
|
Hypertension/Coronary artery disease |
||||
|
No |
14
(40%) |
21
(60%) |
35
(100%) |
1* |
|
Yes |
2 (40%) |
3 (60%) |
5 (100%) |
|
|
Body
mass index>25 kg/m² |
||||
|
No |
14 (41.18%) |
20 (58.82%) |
34 (100%) |
1* |
|
Yes |
2
(33.33%) |
4
(66.67%) |
6
(100%) |
|
† Chi square test.
*
Fisher's exact test.
Table
2: Association
of cephalic venous diameter(mm) and its depth from skin(mm) with AVF failure.
|
Cephalic
venous diameter(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Forearm |
||||
|
Mean
± SD |
2.01
± 0.5 |
2.35
± 0.49 |
2.21
± 0.52 |
0.037‡ |
|
Median(25th-75th percentile) |
1.95(1.7-2.2) |
2.3(2.175-2.525) |
2.2(1.9-2.5) |
|
|
Range |
1.1-3.1 |
1.1-3.6 |
1.1-3.6 |
|
|
Arm |
||||
|
Mean
± SD |
2.51
± 0.54 |
3.4
± 0.73 |
3.04
± 0.79 |
0.0002‡ |
|
Median(25th-75th percentile) |
2.45(2.175-2.725) |
3.3(2.875-3.6) |
2.9(2.575-3.4) |
|
|
Range |
1.7-3.9 |
2.5-5.5 |
1.7-5.5 |
|
|
Depth
from skin(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Mean ± SD |
4.19 ± 1.87 |
3.34 ± 1.32 |
3.68 ± 1.6 |
0.101‡ |
|
Median(25th-75th
percentile) |
4.35(2.75-5.175) |
3.1(2.375-4.625) |
3.3(2.375-4.925) |
|
|
Range |
1.5-8.5 |
1.5-5.5 |
1.5-8.5 |
‡
Independent t test.
Table
3: Association
of artery diameter(mm) , PSV(cm/sec) and intimal wall calcification with AVF
failure.
|
Artery
diameter(mm) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
Radial artery |
||||
|
Mean
± SD |
1.97
± 0.29 |
2.19
± 0.39 |
2.1
± 0.37 |
0.064‡ |
|
Median(25th-75th percentile) |
2(1.8-2.1) |
2.15(1.9-2.325) |
2.1(1.9-2.2) |
|
|
Range |
1.2-2.5 |
1.5-3.1 |
1.2-3.1 |
|
|
Brachial artery |
||||
|
Mean
± SD |
3.34
± 0.6 |
3.79
± 0.72 |
3.61
± 0.7 |
0.046‡ |
|
Median(25th-75th percentile) |
3.15(2.8-3.925) |
3.6(3.475-3.85) |
3.6(3.1-3.925) |
|
|
Range |
2.6-4.4 |
2.9-5.4 |
2.6-5.4 |
|
|
PSV(cm/sec) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|||||
|
Mean ± SD |
55.93 ± 17.04 |
48.38 ± 15.1 |
51.4 ± 16.13 |
0.149‡ |
|||||
|
Median(25th-75th
percentile) |
54 |
45.5 |
51 |
||||||
|
Range |
33-100 |
29-75 |
29-100 |
||||||
|
Intimal
wall calcification in artery |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
||||
|
No |
3 (13.64%) |
19 (86.36%) |
22 (100%) |
0.0003* |
|
||||
|
Yes |
13
(72.22%) |
5
(27.78%) |
18
(100%) |
|
|||||
|
Total |
16 (40%) |
24 (60%) |
40 (100%) |
|
|||||
‡ Independent t test.
*
Fisher's exact test.
Table
4: Association
of fistula blood flow(mL/min) with AVF failure.
|
Fistula
blood flow(mL/min) |
With
AVF failure(n=16) |
Without
AVF failure(n=24) |
Total |
P
value |
|
On day 7 |
||||
|
Mean
± SD |
91.62
± 41.6 |
455.17
± 129.68 |
309.75
± 207.64 |
<.0001‡ |
|
Median(25th-75th percentile) |
82.5(63.75-127.75) |
445(402.25-556.25) |
391(89.25-451.5) |
|
|
Range |
10-154 |
78-670 |
10-670 |
|
|
At 6 weeks |
||||
|
Mean
± SD |
145.41
± 77.76 |
734.79
± 250.25 |
499.04
± 353.22 |
<.0001‡ |
|
Median(25th-75th percentile) |
135.75(100-200.5) |
690(653-768.5) |
608(157-703) |
|
|
Range |
10-290 |
154-1500 |
10-1500 |
|
‡
Independent t test.
Table
5: Receiver
operating characteristic curve of Fistula blood flow(mL/min) on day 7 for
predicting AVF failure.
|
Variables |
Fistula
blood flow(mL/min) on day 7 |
|
Area under the ROC curve (AUC) |
0.978 |
|
Standard
Error |
0.0227 |
|
95% Confidence interval |
0.873 to 1.000 |
|
P
value |
<0.0001 |
|
Cut off |
≤154 |
|
Sensitivity(95%
CI) |
100%(79.4
- 100.0%) |
|
Specificity(95% CI) |
95.83%(78.9 - 99.9%) |
|
PPV(95%
CI) |
94.1%(71.3
- 99.9%) |
|
NPV(95% CI) |
100%(85.2 - 100.0%) |
|
Diagnostic
accuracy |
97.50% |



References
1. Lee T (2013) Novel paradigms for
dialysis vascular access: downstream vascular biology-is there a final common
pathway? Clin J Am Soc Nephrol 8: 2194-2201. [Crossref]
2. Zhu Y, Ding H, Fan PL, Gu QL, Teng J
et al. (2016) Is brachial artery blood flow measured by sonography during early
postoperative periods predictive of arteriovenous fistula failure in
hemodialysis patients? J Ultrasound Med 35: 1985-1992. [Crossref]
3. Wilmink T, Hollingworth L, Powers S,
Allen C, Dasgupta I et al. (2016) Natural history of common autologous
arteriovenous fistulae: consequences for planning of dialysis access. Eur J
Vasc Endovasc Surg 51: 134-140. [Crossref]
4. Yerdel MA, Kesenci M, Yazicioglu KM,
Döşeyen Z, Türkçapar AG et al. (1997) Effect of hemodynamic variables on
surgically created arteriovenous fistula flow. Nephrol Dial Transplant
12: 1684-1688. [Crossref]
5. Lok CE, Huber TS, Lee T, Shenoy S,
Yevzlin AS et al. (2020) KDOQI Clinical Practice Guideline for Vascular Access:
2019 Update. American Journal of Kidney Diseases 75: S1-S164.
6. Indications for initiation of
dialysis in chronic kidney disease.
7. Wong V, Ward R, Taylor J, Selvakumar
S, How TV et al. (1996) Factors associated with early failure of arteriovenous
fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 12: 207-213.
[Crossref]
8. Mendes RR, Farber MA, Marston WA,
Dinwiddie LC, Keagy BA et al. (2002) Prediction of wrist arteriovenous fistula
maturation with preoperative vein mapping with ultrasonography. J Vasc Surg
36: 460-463. [Crossref]
9. Silva MB Jr, Hobson RW 2nd, Pappas
PJ, Jamil Z, Araki CT et al. (1998) A strategy for increasing use of autogenous
hemodialysis access procedures: impact of preoperative noninvasive evaluation. J
Vasc Surg 27: 302-307. [Crossref]
10. Kakkos SK, Kaplanis N, Papachristou
EC, Papadoulas SI, Lampropoulos GC et al. (2017) The Significance of Inflow
Artery and Tourniquet Derived Cephalic Vein Diameters on Predicting Successful
Use and Patency of Arteriovenous Fistulas for Haemodialysis. Eur J Vasc
Endovasc Surg 53: 870-878. [Crossref]
11. Misskey J, Hamidizadeh R, Faulds J,
Chen J, Gagnon J et al. (2020) Influence of artery and vein diameters on
autogenous arteriovenous access patency. J Vasc Surg 71: 158-172.e1. [Crossref]
12. Ren C, Chen J, Wang Y, Huang B, Lu W
et al. (2018) Application of ultrasonography in monitoring the complications of
autologous arteriovenous fistula in hemodialysis patients. Medicine
(Baltimore) 97: e12994. [Crossref]
13. Allon M, Litovsky S, Young CJ,
Deierhoi MH, Goodman J et al. (2013) Correlation of pre-existing vascular
pathology with arteriovenous graft outcomes in hemodialysis patients. Am J
Kidney Dis 62: 1122-1129. [Crossref]
14. Jankovic A, Damjanovic T, Djuric Z,
Marinkovic J, Schlieper G et al. (2017) Calcification in arteriovenous fistula
blood vessels may predict arteriovenous fistula failure: a 5-year follow-up
study. Int Urol Nephrol 49: 881-887. [Crossref]
15. Allon M, Robbin ML, Umphrey HR,
Young CL, Deierhoi MH et al. (2015) Preoperative Arterial Microcalcification
and Clinical Outcomes of Arteriovenous Fistulas for Hemodialysis. Am J
Kidney Dis 66: 84-90. [Crossref]
16. Yap YS, Chi WC, Lin CH, Liu YC, Wu
YW (2021) Association of early failure of arteriovenous fistula with mortality
in hemodialysis patients. Sci Rep 11: 5699. [Crossref]
17. Venkatnarayanan R, Dogra PM,
Bavdekar R, Singh SK, Mondal AK (2020) Primary Failure of Autogenous
Arteriovenous Fistula: Critical Analysis. Indian J Nephrol 30: 382-390.
[Crossref]
18. Yan Y, Ye D,
Yang L, Ye W, Zhan D et al. (2018) A meta-analysis of the association between
diabetic patients and AVF failure in dialysis. Ren Fail 40: 379-383. [Crossref]
19. Malekmakan L, Haghpanah S, Pakfetrat
M, Malekmakan A, Khajehdehi P et al (2009) Causes of chronic renal failure
among Iranian hemodialysis patients. Saudi J Kidney Dis Transpl 20:
501-504. [Crossref]
20. Bahrami Ahmadi A, Zadeh MK,
Chehrehgosha H, Abbasi M (2022) Early Failure of Arteriovenous Fistula (AVF):
The Effect of Diabetes and Hypertension in a Cross-Sectional Study. Med J
Islam Repub Iran 36: 89. [Crossref]
21. Gordon AC, Dholakia S, Ashby D, s
Crane J (2016) Diabetes should not dissuade arteriovenous fistula formation. British
Journal of Diabetes 16: 119-122.
22. Kats M, Hawxby AM, Barker J, Allon M
(2007) Impact of obesity on arteriovenous fistula outcomes in dialysis
patients. Kidney Int 71: 39-43. [Crossref]
23. Sedlacek M, Teodorescu V, Falk A,
Vassalotti JA, Uribarri J et al (2001) Hemodialysis access placement with
preoperative noninvasive vascular mapping: comparison between patients with and
without diabetes. Am J Kidney Dis 38:560-564. [Crossref]
24. Lockhart ME, Robbin ML, Allon M
(2004) Preoperative Sonographic Radial Artery Evaluation and Correlation With
Subsequent Radiocephalic Fistula Outcome. J Ultrasound Med 23: 161-168.
[Crossref]
25. Robbin ML, Greene T, Cheung AK,
Allon M, Berceli SA et al. (2016) Hemodialysis Fistula Maturation Study Group.
Arteriovenous Fistula Development in the First 6 Weeks after Creation. Radiology
279: 620-629. [Crossref]
26. Ladenheim ED, Lulic D, Lumm C,
Agrawal S, Chadwick N (2016) First-week postoperative flow measurments are
highly predictive of primary patency of radiocephalic arteriovenous fistulas. J
Vasc Access 17: 307-312. [Crossref]
27. Shintaku S, Kawanishi H, Moriishi M,
Ago R, Banshodani M et al. (2017) Postoperative day 1 access blood flow and
resistive index can predict patency in distal forearm arteriovenous fistula. J
Vasc Access 18: 371-378. [Crossref]
28. Venkat Ramanan S, Prabhu RA, Rao IR,
Chawla A, Shenoy SV et al. (2022) Outcomes and predictors of failure of
arteriovenous fistulae for hemodialysis. Int Urol Nephrol 54: 185-192. [Crossref]
29. Bylsma LC, Gage SM, Reichert H, Dahl SLM, Lawson JH (2017) Arteriovenous Fistulae for Haemodialysis: A Systematic Review and Meta-analysis of Efficacy and Safety Outcomes. Eur J Vasc Endovasc Surg 54: 513-522. [Crossref]
30. Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ et al. (2011) Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol 6: 1996-2002. [Crossref]
