Robotic Rar for Rectal Cancer with Partial Pelvic Peritonectomy for Carcinosis from Pancreatic Cancer
A B S T R A C T
Introduction: Robotic technology can be used to address several common complications of solid organ malignancies. This case report deals with a patient with rectal cancer undergone robotic rectal resection for bleeding adenocarcinoma, with a new finding of primary pancreatic cancer at post-neoadjuvant re-evaluation.
Materials and Methods: A 70-year-old female patient with adenocarcinoma of the rectum was referred to neoadjuvant treatment. Re-evaluation showed a new finding of a pancreatic tumor. CHT was halted for rectal tumor bleeding after 1 week. Palliative rectal surgery was recommended.
Results: Patient was discharged on 6th postoperative day; CHT was resumed. No pelvic relapse was diagnosed, the pancreatic lesion reduced volumetrically and a RT was performed. After 1 year from surgery the patient referred to our ER for intestinal obstruction. An explorative laparoscopy revealed a diffuse carcinosis from pancreas.
Conclusion: Robotic surgery is safe also in palliative surgery. Minimally-invasive approach minimizes adverse effects of surgical intervention.
Keywords
Palliative surgery, robotic surgery, colorectal cancer, pancreatic cancer, quality of life
Introduction
Palliative care has the goal of preserving and/or improving quality of life in advanced cancer patients with worsening symptoms because of progressive and/or metastatic disease without any opportunities to standard curative-based treatments [1]. In this setting, as oncologic therapies improve advanced cancer patients’ survival, the role of effective surgery for palliation will only increase and robotic surgery may represent the best choice for providing optimal patient comfort in selected cases [2].
In the last years, robotics has increased the choice and access of healthcare for patients and robotic surgery has increasingly diffused widespread, especially in the field of colorectal surgery as it offers a variety of advantages compared to other techniques [3-4].
In this article, we illustrate the case of a patient with rectal cancer undergone neoadjuvant therapy with a new finding of primary pancreatic cancer at post-neoadjuvant re-evaluation and undergone robotic anterior rectal resection for a bleeding adenocarcinoma of the rectum as advanced palliative surgery.
Case Presentation
A 70-year-old female patient presented to the outpatient clinic with diarrhea and rectal bleeding. Preoperative work up showed a rectal tumor at about 8 cm from the anal verge, no pulmonary disease, no carcinosis. Multidisciplinary tumor board recommended long-course neoadjuvant radiotherapy (RT). Post-neoadjuvant work up showed a partial response (PR) disease, but CT scan revealed a neoplasm of the pancreatic body. Ultrasonography and magnetic resonance cholangiopancreatography (MRCP) confirmed a mesopancreatic tumor without a cleavage plane with splenic vessels. Multidisciplinary tumor board recommended a chemotherapy schedule based on gemcitabine, but it was halted for rectal bleeding after 1 week because patient complained of rectorrhagia and severe anemia. A rectoscopy confirmed the bleeding from the rectal lesion. Palliative rectal surgery was recommended.
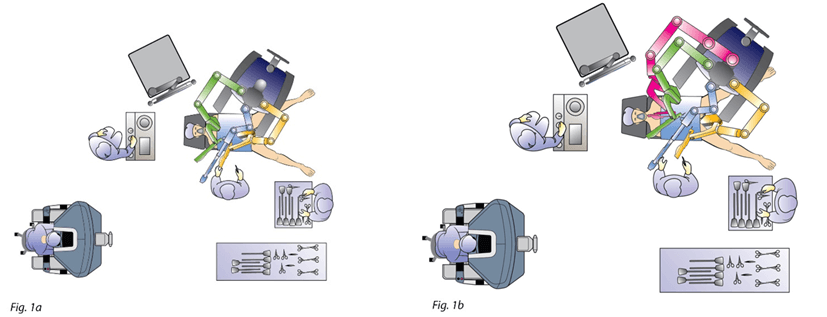
An explorative laparoscopy showed a carcinosis of the pelvic peritoneum and a liver metastasis of the third segment. An intraoperative ultrasonography of the liver didn’t find any other metastases. The procedure was performed by a four-arm da vinci SI surgical system (Intuitive Surgical, Sunnyvale, CA) with a four-arm configuration and a six-port technique. The robot was docked on the left side of the patient following an imaginary line connecting the left anterosuperior iliac spine to the real umbilicus. Patient was placed in anti-trendelenburg position (Figure 1a). The procedure started with the mobilization of the left colic flexure by the supramesocolic approach. The gastrocolic ligament was sectioned in a medial-to-lateral direction, starting from the Bouchet area, granting the access to the omental bursa. Afterwards, the root of the transverse mesocolon was sectioned at the inferior margin of the pancreas thus achieving the mobilization of the left colic flexure.
Figure 1: Operating theater setting and robot docking. a) Robot docking for splenic flexure mobilisation. b) Robot docking and 3rd-arm added for vascular and TME steps. TME: Total Mesorectal Excision.
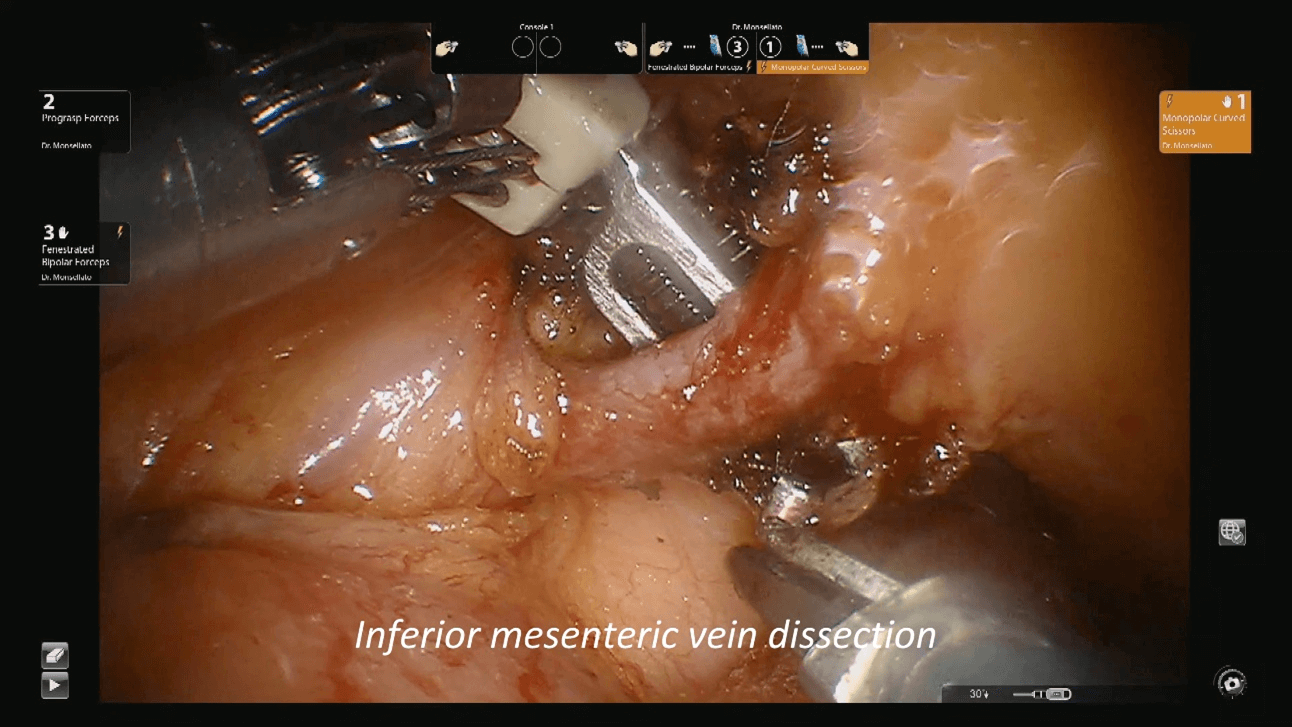
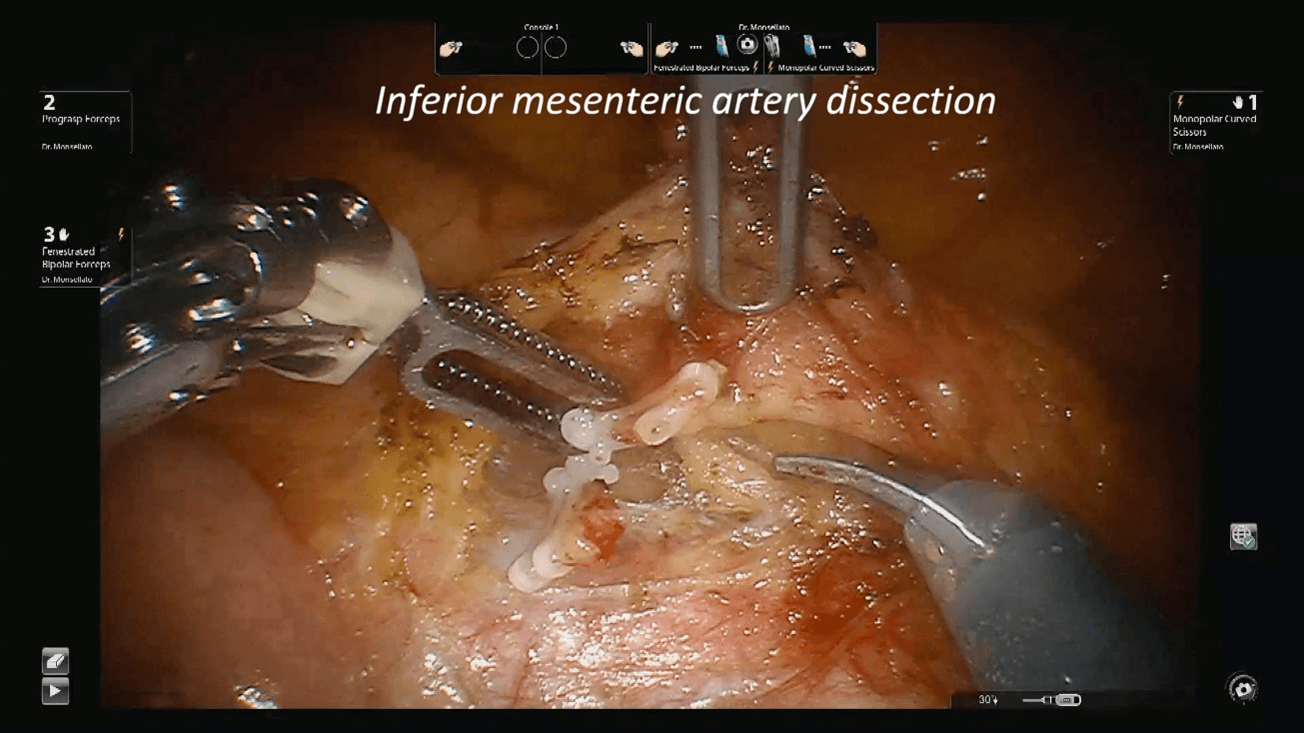
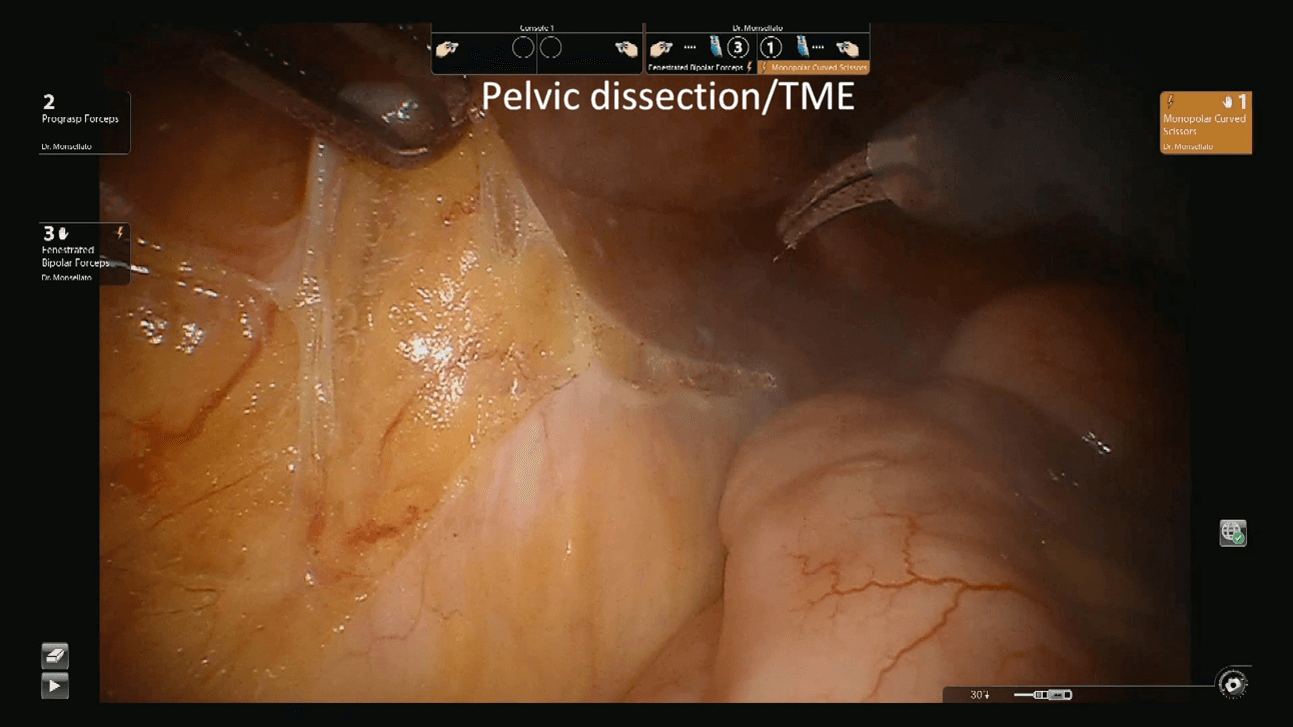
Patients was then placed in trendelenburg position and the robot redocked with the third operative arm (Figure 1b). The inferior mesenteric vein was first approached and resected after its isolation between clips at the level of the inferior margin of the pancreas, as usual (Figure 2). Afterwards, the right pelvic peritoneal sheet was incised, starting from the sacral promontory to the previously dissected area, overexposing the preaortic plane and the origin of the inferior mesenteric artery, that was resected between clips (Figure 3). The dissection continued down to the distal rectum with a total mesorectal excision. The peritoneal pelvic sheet was removed en bloc with the rectum including the carcinosis nodules (Figure 4). The pelvic floor was then reached, and the dissection ended. An indocyanine green test was performed and then the rectum was resected with a linear stapler. A small metastasis of the 3s was also resected. An end-to-end colorectal anastomosis according to the Knight & Griffen technique and diverting ileostomy were fashioned.
Figure 2: Inferior mesenteric vein dissection.
Figure 3: Inferior mesenteric artery dissection. The artery has been cut.
Figure 4: Pelvic dissection and TME. TME: Total Mesorectal Excision.
Patient underwent an anterior rectal resection with S3 metastasectomy and partial pelvic peritonectomy for carcinosis (S3 metastasis and carcinosis were intraoperative findings). Operative time was 273 min with negligible blood loss. Post-operative course was uneventful, and patient was discharged on 6th postoperative day. Histological examination showed a primary rectal adenocarcinoma ypT3 ypN0 (Mandard grade 3). Liver metastasis and pelvic carcinosis were originated from the primary pancreatic tumor.
Chemoradiotherapy was scheduled based on gemcitabine and nab-paclitaxel (9 cycles). Patient underwent ileostomy closure. Re-evaluation with CT scan and MRI showed no metastases and pancreatic PR disease, and a stereotactic radiotherapy was scheduled on the pancreatic lesion. After 1 year from surgery patient referred to our emergency room for intestinal obstruction. A laparoscopic exploration revealed a diffuse carcinosis from pancreatic origin. Patient was addressed to palliative care and after few months died.
Discussion
People diagnosed with cancer are highly heterogeneous in biological characteristics and clinical course. There are the 'survivors', which can be identified as those patients with disease that progresses slowly, often with an acceptable quality of life. But there are also patients who are in a disease condition that can no longer be controlled [5]. When facing the impossibility of curing the disease, does the role of surgery end? When there are no more chances to operate and intervene on patients’ survival, focus should be canalized on increasing their quality of life [6]. The primary aim of palliative intervention is symptoms relief, so clearly the patient has to present certain debilitating indicators, such as: pain, bleeding, obstruction, perforation etc. In addition, specific patient status must be analysed, like his fitting for surgery, comorbidity and other criteria that may have an impact on the outcome of the intervention [6-9]. Surgery exposes to certain risks, then to consider those acceptable and have the benefits related to a palliative surgery, strict criteria in selecting those patients who could benefit from palliative surgery must be chosen [10].
In literature, there are several studies that show how the introduction of robotics has made improvement in terms of outcomes, reduction of intraoperative risks and post-operative complications, so its adoption may reduce surgical stress and guarantee a faster recovery and broaden indications of palliative surgery. Other commonly asserted advantages of the robotic platform could be summarized as follows: improved three-dimensional vision under operator's control, tremor filtering, easier implementation of fine movements that would require higher dexterity and complexity during a laparoscopy [2, 11, 12]. Efficiency of robotic surgery has been testified in many fields, both for curative and palliative purposes.
In their meta-analysis, Guerrini et al. [13] advocated the efficacy, safety, and feasibility of robotic gastrectomy over the laparoscopic approach for patients with gastric cancer. As reported, the results of the study confirmed that robotic technique offers better surgical outcomes and comparable oncological results than conventional laparoscopy. Baek et al. showed a significantly increased number of retrieved lateral pelvic lymph nodes [14].
In other experiences, the use of robotics is not only reported for the treatment of colorectal primary tumor, but also for metastases. Osofsky et al. reported a case of a rare colonic metastatic melanoma, which caused lower gastrointestinal bleeding, treated with robotic surgery. The patient had adequate pain control and a well-tolerated clear-liquid diet in the post-operative and quickly discharged [11]. Bencini et al. reported a case of robotic pancreatic surgery, emphasizing how this minimally invasive approach not only decreased complications, including mortality, but also it allows patients to return to their daily activities in shorter time. From an oncological point of view, faster recovery translates into a shorter interval between surgery and systemic therapy [15]. Same results and advantages are confirmed by Sandozi et al., describing a palliative robotic distal ureterectomy and reimplantation for obstructing metastatic melanoma to the right distal ureter [16].
Even though robotic technique requires higher operating time, it has been seen that it is associated with less intraoperative blood loss and lower rate of surgical complication, and less conversion to open [11, 17]. In our specific case, a critical decision-making was experienced. We faced a bleeding rectal tumor, that altered the algorithm of pancreatic tumor treatment and the pancreatic tumor itself that stopped the treatment of rectal tumor. Once the pancreatic tumor was found, indeed, there was no more indication to continue the treatment with the surgery of rectal tumor as planned. Given the alarming aggressivity and poor survival related to pancreatic cancer, priority was given to its cure. Nevertheless, bleeding of rectal cancer gave back the attention to surgery. At this time, the possibility of a robotic intervention allowed us making a safe choice overcoming the complexity related to the decision-making.
Survival in advanced cases like ours is 8 to 12 months and only three to six months for those with metastatic disease at presentation. Chemotherapy was surely necessary to safeguard and extend, as possible, patient life expectancy, however a palliative surgery was of primary importance to reduce symptom burden. Our intervention allowed patient earning another year of life improving its quality. Although the use of robotics in palliative care is limited, as patients survive longer as it could foresee an increase in its diffusion. Robotic technique could represent a safe and an effective tool for palliative surgery. In complex cases, a robotic technique provides a fast recovery for further oncologic treatments. Palliative surgery, however, must be delivered through a minimally invasive approach to reduce pain and adverse effects of surgical intervention.
Article Info
Article Type
Case ReportPublication history
Received: Tue 26, Sep 2023Accepted: Fri 13, Oct 2023
Published: Fri 03, Nov 2023
Copyright
© 2023 Igor Monsellato. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2023.03.02
Author Info
T. Gatto G. Costantini A. Chahrour Igor Monsellato
Corresponding Author
Igor MonsellatoAzienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Divisione di Chirurgia Generale ad Indirizzo Oncologico, Alessandria, Italy
Figures & Tables
References
1. Cloyd JM (2019) Minimally Invasive Surgery for
Palliation. Surg Oncol Clin N Am 28: 79-88. [Crossref]
2. Nwosu AC, Sturgeon B, McGlinchey T, Goodwin CD, Behera
A et al. (2019) Robotic technology for palliative and supportive care:
Strengths, weaknesses, opportunities and threats. Palliat Med 33: 1106-1113. [Crossref]
3. D'Annibale A, Pernazza G, Morpurgo E,
Monsellato I, Pende V et al. (2010) Robotic right colon resection: evaluation of first 50 consecutive cases
for malignant disease. Ann Surg Oncol 17: 2856-2862. [Crossref]
4. Monsellato I, Morello A, Prati M, Argenio G, Piscioneri D et al. (2019) Robotic transanal total mesorectal excision: A new
perspective for low rectal cancer treatment. A case series. Int J Surg Case
Rep 61: 86-90. [Crossref]
5. I numeri del cancro 2022 AIOM-Registri Tumori Italiani SIAPEC-PASSI-PASSI
D’ARGENTO-ONS-Fondazione AIOM.
6.
Goel
A, Garg PK (2018) The Role of Surgery in Palliative Care of Cancer: A Review. Gulf
J Oncolog 1: 61-71. [Crossref]
7.
Sun
V, Krouse RS (2014) Palliative surgery: incidence and outcomes. Semin Oncol
Nurs 30: 234-241. [Crossref]
8.
McCahill
L, Ferrell B (2002) Palliative surgery for cancer pain. West J Med 176:
107-110. [Crossref]
9.
Nevo
Y, Morency D, Kammili A, Abdrabo L, Zullo K et al. (2022) The Role of
Palliative Surgery in Stage IV Gastric Cancer: A Retrospective Study. J
Palliat Care 37: 152-158. [Crossref]
10.
Foster
D, Shaikh MF, Gleeson E, Babcock BD, Ringold D et al. (2016) Palliative Surgery
for Advanced Cancer: Identifying Evidence-Based Criteria for Patient Selection:
Case Report and Review of Literature. J Palliat Med 19: 22-29. [Crossref]
11.
Osofsky
R, Kamya C, Hanif H, Phuoc V (2021) A rare cause of lower gastrointestinal
bleeding treated with robotic colorectal surgery. Surg Case Rep 7: 125.
[Crossref]
12. Williamson T, Song
SE (2022) Robotic Surgery Techniques to Improve Traditional Laparoscopy. JSLS 26: e2022.00002. [Crossref]
13.
Guerrini GP, Esposito
G, Magistri P, Serra V, Guidetti C et al. (2020) Robotic versus laparoscopic
gastrectomy for gastric cancer: The largest meta-analysis. Int J Surg
82: 210-228. [Crossref]
14.
Baek
SJ, Piozzi GN, Kim SH (2021) Optimizing outcomes of colorectal cancer surgery
with robotic platforms. Surg Oncol 37: 101559. [Crossref]
15.
Bencini
L, Urciuoli I, Trafeli M, Paolini C, Moraldi et al. (2021) Robotic pancreatic
surgery: minimally invasive approach to challenging operations. Minerva Surg
76: 138-145. [Crossref]
16. Sandozi A, Jivanji D, Huang Y, Azar O, Schulman A (2022) Robotic distal ureterectomy and re-implant for obstructing metastatic melanoma - surgical considerations in the palliative setting. Can J Urol 29: 11323-11325. [Crossref]
17. Spinoglio G, Bianchi PP, Marano A, Priora F, Lenti LM et al. (2018) Robotic Versus Laparoscopic Right Colectomy with Complete Mesocolic Excision for the Treatment of Colon Cancer: Perioperative Outcomes and 5-Year Survival in a Consecutive Series of 202 Patients. Ann Surg Oncol 25: 3580-3586. [Crossref]
