Journals
Role of the complete pathological response in rectal cancer: Value as a prognostic factor
A B S T R A C T
Objective
To study the effect of the pathologic complete response (pCR) on the survival of patients treated with surgery and neoadjuvant chemo-radiotherapy in locally advanced non-metastatic rectal carcinoma (LARC).
Materials and methodology
We underwent an observational retrospective analysis of cohorts. The recruitment was carried out by means of non-probabilistic consecutive inclusion of patients with rectal cancer treated between January 2009 and December 2016 with surgery and neoadjuvant chemo-radiotherapy. The patients recruited had been diagnosed with locally advanced non-metastatic rectal cancer. cT3-4 o N+. based on the American Joint Committee on Cancer (AJCC) 2010. with histological confirmation of adenocarcinoma and no treatment with induction chemotherapy. The pathologic response was calibrated in accordance with the Ryan system. Survival was calculated with multivariate Cox regression analysis
Results
Pathologic complete response was reached by 19.2% Patients. The disease free survival was significantly lower in the no pathologic complete response (HR 0.099. p value 0.025). The progression in the group of patients with pathological complete response occurred in only one patient and have local and distal component compared to 39 patients in no pCR 21.2% distant metastases and 3.8% locally relapse.
Perineural invasion and adjuvant chemotherapy were also significatly associated with disease free survival.
Conclusions
The pathological complete response is a good prognosis factor in patients treated with surgery and nCRT in LARC with distal and local relapse. Perineural invasion and adjuvant chemotherapy were also good prognostic factors.
Keywords
Rectal carcinoma, survival neoadjuvant chemo-radiotherapy surgery
Abbreviations
AJCC: American Joint Committee on Cancer
CEA: Carcinoembryonic antigen
LARC: locally advanced non-metastatic rectal carcinoma
nCRT: Neoadjuvant chemo-radiotherapy
pCR: pathologic complete response
SD: Standard deviation
TME: Total mesorectal excision
I N T R O D U C T I O N
Colorectal cancer is a significant health problem at global level, with an estimated 1.8 million newly diagnosed cases in 2018 and 881.000 deaths, it being third in terms of incidence and second in terms of mortality. It is estimated that in 2018 there have been 430.230 cases of rectal cancer in males and 274.146 in females [1]. In Spain there is no register of tumour occurrence at national level. The Community of Madrid has its own record, the data published in 2017 showing that gastro-intestinal tumours were recorded as the most frequent, comprising 23.2% of the total, with 997 cases of rectal cancer diagnosed throughout that year [2].
Surgery is the only curative treatment for locally advanced non-metastatic rectal carcinoma, being total mesorectal excision (TME) the gold standard treatment for this disease, taking into account that the rate of local recurrences is between 4% and 27% while that of lymph node involvement can reach 15% [3].The most appropriate surgical technique is selected based on how far the tumour is from the anal margin as well as the clinico-radiological status. The carcinomas which are located in the upper or middle third of the rectum tend to require intervention by means of low anterior resection while only those tumours which do not have a 2 cm margin of healthy tissue distal to the tumour, will be treated by means of abdomino-perineal resection.
In spite of the improvement in results with TME, neoadjuvant chemo-radiotherapy (nCRT) is also a very important aspect in the successful treatment of such patients. Neoadjuvant chemo-radiotherapy demonstrated less toxicity (27% vs 40% p 0.001) and a lower rate of local recurrence (6% vs 13% p 000.6) than post-operative, without impacting on global survival and nowadays it is considered in the majority of the guidance as the standard approach in locally advanced non-metastatic rectal cancer [4-6].
At the moment there is no consensus in regard to adjuvant treatment, the literature being contradictory. However, the guidance recommends postoperative treatment based on 5-fluorouracil, this treatment being extrapolated from studies of colon cancer [10]. In the field of oncology there is currently a multitude of studies being carried out in order to assess the range of prognosis factors to help determine the best treatment to select for patients, taking into account the individual risk. In rectal cancer the clinical stage at diagnosis is a factor for prognosis of survival to 5 years, being for stage II from 60% to 75% and for stage III from 40% to 60%. Treatment with neoadjuvant chemo-radiotherapy produces a wide range of responses in the tumour, the best responding patients having the better prognosis, above all those who achieve the pathologic complete response (pCR) [11-13]. There are a number of systems used to classify the responses, the Ryan system published in 2005 being that used by the American College of Pathologists (Table 1) [14]. Other factors have been studied which may also help forecast the survival of patients and help determine the best treatment [15-19]. Some of these are pathological such as lympho-vascular invasion and perineural infiltration while others are clinical factors such as the carcinoembryonic antigen (CEA) [15-17].
Table 1
|
Description |
|
|
No viable cancer cells (pCR) |
0 |
|
Single cells or small groups of cancer cells (near complete response) |
1 |
|
Residual cancer with evident tumor regression. but more than single cells or rare small groups of cancer cells (partial response) |
2 |
Our objective was to evaluate the effect of the response (pCR) in survival of the patients with locally advanced rectal carcinoma treated with surgery and neoadjuvant chemo-radiotherapy and the pattern of recurrence in this subgroup of patients.
Materials and methods
A retrospective observational study of cohorts was carried out, the participants having been recruited by non-probabilistic consecutive sampling of patients with rectal cancer treated with neoadjuvant chemo-radiotherapy at Hospital Universitario de Fuenlabrada and Hospital Fundación Alcorcón between January 2009 and December 2016. The study was endorsed by the Ethics Committee of both hospitals from which the participants were selected.
Those recruited were patients who had been diagnosed with locally advanced non-metastatic rectal carcinoma, CT3-4 o N+, according to the American Joint Committee on Cancer (AJCC) in 2010, stages II-III, with histological confirmation of adenocarcinoma and who had not been treated with induction chemotherapy. A sample size was calculated according to a reliability level of 80% (error Type I of 5%), a statistical power of 80%, expected survival of 50% and a loss in monitoring of 5%. Thus, it was estimated that 190 patients would be required. All the patients underwent surgery and determination of CEA on diagnosis was carried out, measurement of the anal margin via rectoscopy, CT of the pelvic-abdomino thorax for extended studies and pelvic MRI for local staging.
Radiotherapy
All the patients received radiotherapy consisting of 25 fractions of 1.8 Gy per fraction administering a total dosage of 45 Gy CTV pelvic and subsequently 3 fractions of 1.8 Gy sequentially until reaching an additional dose of 5.4 Gy to the tumour and macroscopically suspicious adenopathy. Intestinal extraction techniques used were by means of extrinsic compression and full bladder (prone position, bellyboard use). In all patients, the prophylactic CTV included the mesorrectum, posterior pelvic wall and internal iliac lymph nodes. The lower pelvis included tumours of < 6 cm from the anal margin or affecting the sphincter or which were subject to abdomino-perineal resection. The external iliac groups only if there was involvement of the pelvic organs (uterus, bladder, vagina, prostate, urethra) and the inguinal groups exclusively in tumours with involvement of the external anal sphincter or of the inferior third of the vagina.
Concomitant chemotherapy
Concomitant chemotherapy comprised two different regimes in accordance with the choice of the medical oncologist given the equal effectiveness of both, the only difference being the toxicity profiles [20] consisting of 5 Fluorouracil (5 FU) in continuous infusion 225 mg/m2 per day or concomitant Capecitabine, 825 mg/m2 every 12 hours orally, taken concurrently with the radiotherapy treatment.
Adjuvant chemotherapy
Due to the fact that there are no uniform criteria in the literature about the role of adjuvant chemotherapy, this is determined by the medical oncologist, the three alternatives used to be: monitoring only, chemotherapy based on 5FU or combinations of this with Oxaliplatin.
Surgery
Surgery was carried out between 6 and 10 weeks following the end of the neoadjuvant treatment, by two different surgeons, both with experience in treatment of colorectal cancer.
Pathological anatomy
The surgical specimens were evaluated by two pathology services. The degree of tumour response being included in all the reports based on the Ryan classification system [14]. The classification and pathology in terms of lymph node involvement, the integrity of the mesorectum, the involvement of the circumferential margin (a margin of less than 1 mm being considered as affected), lymph nodes extracted, lympho-vascular and perineural infiltration.
Statistics
The categorical variables are described as frequency or percentages and compared with the Chi square tests or exact Fisher test. The quantitative variables are described using the average and standard deviation (SD) or average and percentile 25 and 75 and were compared with the Student’s t test after evaluating its normal distribution with the Shapiro-Wilk test. Evaluation of the effects of treatment for time variables until recurrence or time until death was carried out with Kaplan Meier curves and compared with the Logrank test. The parameters which were significant in the univariate model were carried out to the multivariate model using the Cox regression. All the contrasts were bilateral, with p < 0.05, as a cut off point for statistical significance. Analysis was carried out by means of the SPSS statistical package (version 20.0).
Results
Analysis was carried out on 193 patients, with an average age of 63 years (SD 9) and who had been diagnosed and treated for rectal cancer stage II/III between the years 2009 and 2016. Clinical factors of the series showed that 79.34% of the patients were cT3 and 12.4% cT4 (Table 2). In 92.7% of them there was clinical suspicion of lymph node involvement on diagnosis and in 24% there was a combination with elevated CEA. The distribution of tumour location with regard to the distance from the anal margin was 32.4% high tumours (12 to 8 cm), the same percentage for medium (from 8 to 5 cm) while 35.4% were low tumours (distance of 5 cm from the lower margin). After 8 weeks from the date when the radiotherapy ended 66% of surgical procedures were carried out, 73.4% of the patients having low anterior resection and 26.6% abdomino-perineal resection. Pathologic complete response was achieved by 19.2% of the patients. Adjuvant chemotherapy was the treatment of choice in 83.9% of the series, 40.9 % receiving therapies derived from 5 FU and 43% in combination with Oxaliplatin, while 16% were subject to monitoring only.
Table 2: Comparision of pretreatment characteristic
|
|
no pCR |
pCR |
p-value |
|
Pretreatment CEA level (ng/ml) |
|
|
0.392 |
|
< 5 |
74.4% |
81.1% |
|
|
≥ 5 |
25.6% |
18.9% |
|
|
Clinical stage |
|
|
0.47 |
|
II |
6.40% |
10.80% |
|
|
III |
93.60% |
89.20% |
|
|
Distance from the anal verge (cm) |
|
|
0.69 |
|
0-5 |
32.30% |
32.40% |
|
|
> 5 < 8 |
33.50% |
27% |
|
|
> 8 |
34.20% |
40.50% |
|
The average length of monitoring of the whole cohort was 89 months (SD 2.3). On the date of analysis 78.6% of patients were free from disease, the disease-free survival being 95.3 months (SD 2.3) in the group which reached the pCR, as opposed to 76.5 months (SD 3.4) for the rest of the patients (p 0.002), with a difference in global survival of 92.9 (SD 3.2) months as opposed to 87.4 (SD 2.8) months (p=0.125). In the univariate analysis the pre-operative CEA level, adjuvant chemotherapy, pCR, pathological stage, lymphovascular invasion, perineural invasion and the surgical margins were independently associated with disease free survival. Following the multivariate analysis by means of the Cox regression, pCR, perineural infiltration and adjuvant chemotherapy were independent variables in relation to disease free survival following neoadjuvant chemo-radiotherapy (Table 3). In the pCR group there was only one recurrence and it was mixed, local and distal. In the patients who did not achieve pCR, 21.2% developed metastases and 3.8% local recurrence.
Table 3
|
Univariant logistic regresión DFS |
Multivariante logistic regresion |
|||
|
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
|
Adyuvant chemotherapy |
3.49 (1.77-6.8) |
0.00001 |
4.5 (2.2-9.22) |
0.0001 |
|
Compliance chemotherapy |
0.7 (0.2-2.3) |
0.56 |
||
|
Oxaliplatin |
1.3 (0.6-2.7) |
0.48 |
||
|
Clinical stage |
1.7 (0.4-7) |
0.427 |
||
|
Pathological stage |
2.9 (1.5-5.3) |
0.001 |
||
|
Linfovascular invasion |
3 (1.6-5.7) |
0.001 |
||
|
Perineural invasion |
5.3 (2.8-10) |
0.00001 |
3.36 (1.7-6.5) |
0.0001 |
|
Resection margin |
4.3 (1.6-11) |
0.002 |
0.099 (0.013-0.75 |
|
|
Pathological complete response |
0.87 (0.012-0.63) |
0.016 |
0.025 |
|
|
CEA |
1.2 (0.98-1,04) |
0.12 |
||
Discussion
Treatment with pre-operative chemo-radiotherapy in cancer of the rectum has a wide range of outcomes, from poor responses with practically complete persistence of the tumour to the disappearance of all viable tumour cells in the surgical specimen [12, 21, 22]. A number of studies have been published which link these responses to disease-free survival and global survival in these patients [13, 23, 24]. Many studies do not demonstrate these findings and their authors relate this to a positive prognosis in these patients and thus the high number of censored cases in the series published.
This variability in responses mean that there is considerable work to be done in attempting to determine which factors could help us in forecasting and creating models for guidance in decision making [15, 25]. Based on all this, new methods of investigation of rectal carcinoma are being established, such as evaluation of whether it is possible to preserve the organ, some authors suggesting surgery may be avoided in patients who achieve pCR due to their excellence prognosis [26]. However, at this point there is insufficient scientific evidence for conservative treatment to be considered as standard, outside clinical studies with strict monitoring protocols. It is known that the increase in time between the completion of adjuvant treatment and surgery can have a positive impact on increasing the percentage of pCR [25, 26]. In our series 66% of the patients were operated on beyond 8 weeks from the completion of radiotherapy.
The patterns of recurrence in pCR are more favourable than in the partial responses, the most frequent being distant recurrence [12-13, 23-24]. The first place of recurrence is not normally the liver as usually occurs in the majority of the partially responsive patients, something which should be taken into account when selecting the tests for follow up. In our study, perineural infiltration was consolidated as an independent factor in recurrence, findings previously published [16, 27] and which are currently of vital importance when it comes to deciding on adjuvant treatments such as for cancer of the colon. In rectal cancer they have special relevance when the surgical specimen has fewer than isolated lymph glands, this being considered as a factor for radiological resistance or greater biological aggression.
The role of adjuvant chemotherapy is a subject which is very much debated in the literature. There is currently a lack of clarity about the role of adjuvant treatment following chemo-radiotherapy, in terms of whether this should be administered to all patients or whether selection should be made using prognosis factors, nor are those factors defined. All the guides recommend that their use is based on 5 FU, without it being clear what the role of Oxaliplatin is in this scenario [28-36]. It appears that there exists a benefit to its use but without it being determined what the best regime is and what variables we should take into account. However due to the limitations in clinical staging many authors base their decisions on post-operative staging.
Our study does have some limitations. The principal of these is that as it is a retrospective study, it may be affected by selection bias, with exclusion of some patients. The adjuvant chemotherapy schemes were not controlled by the researcher, rather the selection was based on the criteria used by the supervising medical oncologist, although it is true that in the statistical analysis there were no differences between the two groups in the schemes selected.
Conclusions
The best pCR improves the disease-free survival, without increasing global survival rates. In our study, perineural invasion and administration of adjuvant chemotherapy were additional factors which were independent from disease free survival. These findings should be taken into account in order to generate predictive models which could in the future lead us to be able to personalise treatments and intensify these in patients with poor prognoses.
Attempts are being made to increase pCR with intensive neoadjuvant treatments, but it should still be demonstrated whether the patients who have received these different regimens of neoadjuvant treatment maintain the same survival rates as those which has been achieved with classic regimens. The objectives of preserving the organ are based on achievement of the response and should continue to be monitored within clinical studies, there being no current observation of a standard treatment of these patients.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 14, Dec 2018Accepted: Sun 30, Dec 2018
Published: Thu 10, Jan 2019
Copyright
© 2023 Gil Rodríguez-Caravaca. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2018.01.005
Author Info
Gil Rodríguez-Caravaca Ignacio Juez-Martel Ma Victoria De-Torres-Olombrada Manuel Duran-Poveda
Corresponding Author
Gil Rodríguez-CaravacaUnit of Preventive Medicine, Hospital Universitario Fundación Alcorcón. Madrid
Figures & Tables
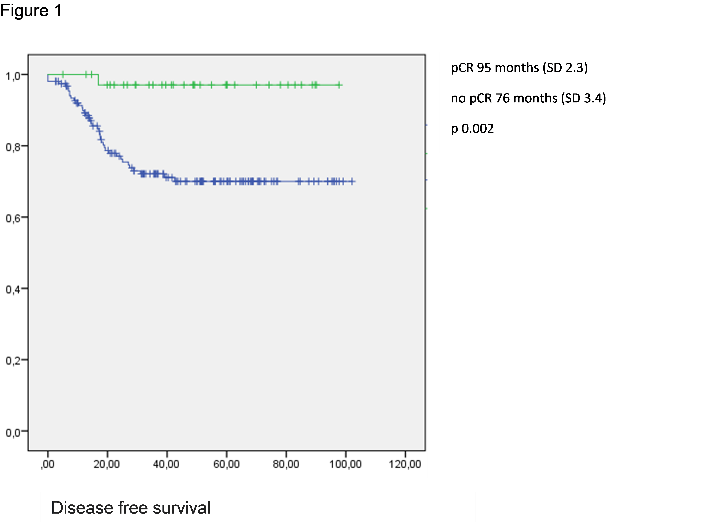
Table 1
|
Description |
|
|
No viable cancer cells (pCR) |
0 |
|
Single cells or small groups of cancer cells (near complete response) |
1 |
|
Residual cancer with evident tumor regression. but more than single cells or rare small groups of cancer cells (partial response) |
2 |
Table 2: Comparision of pretreatment characteristic
|
|
no pCR |
pCR |
p-value |
|
Pretreatment CEA level (ng/ml) |
|
|
0.392 |
|
< 5 |
74.4% |
81.1% |
|
|
≥ 5 |
25.6% |
18.9% |
|
|
Clinical stage |
|
|
0.47 |
|
II |
6.40% |
10.80% |
|
|
III |
93.60% |
89.20% |
|
|
Distance from the anal verge (cm) |
|
|
0.69 |
|
0-5 |
32.30% |
32.40% |
|
|
> 5 < 8 |
33.50% |
27% |
|
|
> 8 |
34.20% |
40.50% |
|
Table 3
|
Univariant logistic regresión DFS |
Multivariante logistic regresion |
|||
|
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
|
Adyuvant chemotherapy |
3.49 (1.77-6.8) |
0.00001 |
4.5 (2.2-9.22) |
0.0001 |
|
Compliance chemotherapy |
0.7 (0.2-2.3) |
0.56 |
||
|
Oxaliplatin |
1.3 (0.6-2.7) |
0.48 |
||
|
Clinical stage |
1.7 (0.4-7) |
0.427 |
||
|
Pathological stage |
2.9 (1.5-5.3) |
0.001 |
||
|
Linfovascular invasion |
3 (1.6-5.7) |
0.001 |
||
|
Perineural invasion |
5.3 (2.8-10) |
0.00001 |
3.36 (1.7-6.5) |
0.0001 |
|
Resection margin |
4.3 (1.6-11) |
0.002 |
0.099 (0.013-0.75 |
|
|
Pathological complete response |
0.87 (0.012-0.63) |
0.016 |
0.025 |
|
|
CEA |
1.2 (0.98-1,04) |
0.12 |
||
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R. Torre L. et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424. [Crossref]
2. Memoria Registro de Tumores de Madrid (RETMAD) (2016) Oficina Regional de Coordinación Oncológica 1-17.
3. Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1: 1479-1482. [Crossref]
4. Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, et al. (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 24: 4620-4625. [Crossref]
5. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, et al. (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30: 1926-1933. [Crossref]
6. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, et al. (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol 15: 184-190. [Crossref]
7. González-Flores E, Losa F, Pericay C, Polo E, Roselló S, et al. (2016) SEOM Clinical Guideline of localized rectal cancer (2016). Clin Transl Oncol 18: 1163-1171. [Crossref]
8. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, et al. (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis. treatment and follow-up. Ann Oncol. [Crossref]
9. NCCN Guidelines (2018) Rectal Cancer.
10. de Gramont A, Tournigand C, Andre T, Larsen AK, Louvet C (2007) Adjuvant therapy for stage II and III colorectal cancer. Semin Oncol 34: 37-40. [Crossref]
11. Mancini R, Pattaro G, Diodoro MG, Sperduti I, Garufi C, et al. (2018) Tumor Regression Grade After Neoadjuvant Chemoradiation and Surgery for Low Rectal Cancer Evaluated by Multiple Correspondence Analysis: Ten Years as Minimum Follow-up. Clin Colorectal Cancer 17: 13-19. [Crossref]
12. Xu L, Cai S, Xiao T, Chen Y, Qiu H, et al. (2017) Prognostic significance of tumour regression grade after neoadjuvant chemoradiotherapy for a cohort of patients with locally advanced rectal cancer: an 8-year retrospective single-institutional study. Colorectal Dis 19: 263-271. [Crossref]
13. Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, et al. (2012) Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 19: 2822-2832. [Crossref]
14. Ryan R, Gibbons D, Hyland JMP, Treanor D, White A, et al. (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47: 141-146. [Crossref]
15. Chapman BC, Hosokawa P, Henderson W, Paniccia A, Overbey DM, et al. (2017) Impact of neoadjuvant chemoradiation on perioperative outcomes in patients with rectal cancer. J Surg Oncol 115: 1033-1044. [Crossref]
16. Lino-Silva LS, Salcedo-Hernández RA, España-Ferrufino A, Ruiz-García EB, Ruiz-Campos M, et al. (2017) Extramural perineural invasion in pT3 and pT4 rectal adenocarcinoma as prognostic factor after preoperative chemoradiotherapy. Hum Pathol 65: 107-112. [Crossref]
17. Huaman MA, Fiske CT, Jones TF, Warkentin J, Shepherd BE, et al. (2015) HHS Public Access 143: 951-952.
18. Valentini V, Van Stiphout RGPM, Lammering G, Gambacorta MA, Barba MC, et al. (2011) Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of european randomized clinical trials. J Clin Oncol 29: 3163-3172. [Crossref]
19. Valentini V, Van Stiphout RGPM, Lammering G, Gambacorta MA, Barba MC, et al. (2015) Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother Oncol 114: 302-309. [Crossref]
20. Zou X Cai, Wang Q Wen, Zhang J Min (2017) Comparison of 5–FU-based and Capecitabine-based Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: A Meta-analysis. Clin Colorectal Cancer 16: 123-139. [Crossref]
21. Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12: 19-23. [Crossref]
22. Jäger T, Neureiter D, Urbas R, Klieser E, Hitzl W, et al. (2017) Applicability of American Joint Committee on Cancer and College of American Pathologists Regression Grading System in Rectal Cancer. Dis Colon Rectum 60: 815-826. [Crossref]
23. Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, et al. (2008) Prognostic Value of Pathologic Complete Response After Neoadjuvant Therapy in Locally Advanced Rectal Cancer: Long-Term Analysis of 566 ypCR Patients. Int J Radiat Oncol Biol Phys 72: 99-107. [Crossref]
24. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, et al. (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 11: 835-844. [Crossref]
25. Letaief F, Nasri M, Ayadi M, Meddeb K, Mokrani A, et al. (2017) Potential predictive factors for pathologic complete response after the neoadjuvant treatment of rectal adenocarcinoma: a single center experience. Cancer Biol Med 14: 327-334. [Crossref]
26. Sun Y, Chi P, Lin H, Lu X, Huang Y, et al. (2017) A nomogram predicting pathological complete response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer : implications for organ preservation strategies. Oncotarget 8: 1-12. [Crossref]
27. Reig Castillejo A, Membrive I, Foro P, Quera J, Sanz X, et al. (2017) Predictive factors for survival in neoadjuvant radiochemotherapy for advanced rectal cancer. Clin Transl Oncol 19: 853-857. [Crossref]
28. Benson AB Rd (2016) Should We Consider Adjuvant Therapy for Rectal Cancer After Neoadjuvant Chemoradiotherapy? Clin Adv Hematol Oncol 14: 778-781. [Crossref]
29. Breugom AJ, Bastiaannet E, Boelens PG, Iversen LH, Martling A, et al. (2016) Adjuvant chemotherapy and relative survival of patients with stage II colon cancer - A EURECCA international comparison between the Netherlands. Denmark. Sweden. England. Ireland. Belgium. and Lithuania. Eur J Cancer 63: 110-117. [Crossref]
30. Loree JM, Kennecke HF, Lee-Ying RM, Goodwin RA, Powell ED, et al. (2018) Impact of Postoperative Adjuvant Chemotherapy Following Long-course Chemoradiotherapy in Stage II Rectal Cancer. Am J Clin Oncol 41: 643-648. [Crossref]
31. Polanco P, Huerta S (2018) Omitting adjuvant chemotherapy in patients with rectal cancer who received neoadjuvant chemoradiation followed by total mesorectal excision and achieved a pathological complete response. Am J Surg 216: 387-388. [Crossref]
32. Lichthardt S, Zenorini L, Wagner J, Baur J, Kerscher A, et al. (2017) Impact of adjuvant chemotherapy after neoadjuvant radio- or radiochemotherapy for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 143: 2363-2373. [Crossref]
33. Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev . [Crossref]
34. Kumar A, Peixoto RD, Kennecke HF, Renouf DJ, Lim HJ, et al. (2015) Effect of Adjuvant FOLFOX Chemotherapy Duration on Outcomes of Patients with Stage III Colon Cancer. Clin Colorectal Cancer 14: 262-268. [Crossref]
35. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, et al. (2015) Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre. phase 2 trial. Lancet Oncol 16: 957-966. [Crossref]
36. Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, et al. (2017) Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified? Am J Surg 213: 478-483. [Crossref].
