SARS-Cov-2 Natural or Artificial? That is the Question
A B S T R A C T
The COVID-19 pandemic has become the most serious health problem of the 21st century. Knowing whether its origin was natural or artificial is an extremely important fact, especially due to the ethical debate that should be generated on gain-of-function investigations in high-security laboratories around the world. We analyse all the existing evidence on the possible origin of the SARS-CoV-2 trying to join the pieces and thus obtain an overview that allows us to see beyond the isolated publications.
Keywords
SARS-Cov-2, COVID-19, natural, artificial, origin, spike protein
Introduction
When the initial outbreak of this pandemic is over, the SARS-Cov-2 (or SARS-nCoV) virus will have infected more than 15 million people, killed more than 750 thousand people (including probably subsequent waves), and destroyed the economy of almost the entire world. planet. That is why knowing with absolute precision how this virus originated and passed the barrier of animals to humans is a matter of global importance. Since the beginning of the year rivers of ink have flowed over the possible origin of SARS-CoV-2. A few days after the start of the infection in China, the Wuhan BSL-4 level laboratory released the complete genome of the virus and since then, many researchers around the world have put their magnifying glasses on this genetic sequence [1]. revealing all its close relatives and looking for the best candidate to be its last host but, unlike all previous epidemics, in this case said animal is proving impossible to find [2-8].
We will probably never know whether the COVID-19 pandemic originated from an artificially created virus or was the product of the natural mutation. I have studied in detail the publications that support both theories and neither of them provides clear and decisive evidence in one way or another. It is important to mention that none of the scientific articles, which propose a possible artificial origin of the virus, presuppose that it has been intentionally released by the Chinese government. It is not fair to try to conspiracy to anyone who dares to hypothesize a possible artificial origin since that possibility is real and the possibility that there was an accidental leak of biological material is not zero. Our duty as scientists is to analyse all the existing arguments about it instead of denying it in a prejudiced way. The Wuhan Laboratory is the center with the highest level of biological danger in China and its experimentation aimed at the potentiation of different viruses and the creation of chimeras (new hybrid microorganisms formed by genetic material of 2 or more different microorganisms) is well known. This fact is perfectly demonstrated by the publications made by said research center [9-26].
The SARS-CoV-2 Virus: General Characteristics and Approach to its Genealogy
It is a new virus that has been classified in the Family: Coronaviridae, Subfamily: Orthocoronaviridae, Genus: Beta Coronavirus, Subgenus: Sarbecovirus. Alphacoronaviruses and betacoronaviruses infect mammals only and are normally responsible for respiratory infections in humans and gastroenteritis in animals [2]. As of SARS-CoV-2, only six coronaviruses had been described in humans (HCoV-NL63, HCoV-229E, HCoV-OC43 and HKU1). The SARS-COV-2 genetic material is structured forming 6 ORFs (Open Reading Frames), identical to the rest of the coronavirus, in addition to several additional genes. Its genome is made up of a single-stranded RNA of positive polarity and almost 30,000 nucleotides that encodes four structural proteins: S (spike protein), E (envelope), M (membrane) and N (nucleocapsid). Protein N is inside the virion associated with viral RNA, and the others are associated with the viral envelope [2].
Protein S on the surface of coronaviruses is responsible for its binding to the cellular receptor and the fusion process with its membrane, making it a determining factor in tropism and transmission capacity in a new host. Furthermore, it is the viral antigen most intensely recognized by the host's immune system and therefore of great importance in the host's defensive response [6, 27]. For protein S to exercise its function, it must be hydrolyzed by lung proteases, giving rise to fragment S1, responsible for binding to the receptor, and fragment S2, responsible for the fusion process to the cell membrane. Even though SARS-CoV and SARSCoV-2 are found in different genetic lineages, they have around 50 amino acids conserved in position S1, while most of those from bats show important antigenic variations in this sensitive area. More specifically, the ability of the S1 protein to bind to the cell is in the C-terminal domain of the cell [8, 28].
In other words, we have a bat virus with an enormous similarity to SARS-CoV-2 but that is incapable of crossing the interspecies barrier and infecting us since its protein S could not effectively bind to the receptor angiotensin-converting enzyme 2 (ACE2) of the human cell membrane. For this reason, it is known with certainty that the virus did not jump directly from the bat to the human and it was necessary to pass it through another intermediate host (pangolin, civet, snake...). In fact, using powerful computers and complex statistical methods, Dr. Trevor Bedford of the University of Washington studies the expansion and evolution of viruses. When analysing SARS-CoV-2, it states that although it is very similar to CoV RaTG13, it places both viruses between 20 and 65 years of evolutionary distance [29].
In Search of the Last Animal Host before Jumping to Humans
I Bat, Pangolin, Civet, Snake... Who is Who?
Globally, when studying the genome of all coronaviruses, SARS-COV-2 has a 79% homology with SARS-CoV and 50% with MERS but the similarity with the BatCoV RaTG13 virus, found in the common bat of Yunnan (Rhinolophus affinis) is 96%, so it is considered as its closest ancestor to date [8]. However, the RBD area of protein S differs significantly. Metagenomic analyses have shown the presence of viral genetic sequences of the pangolin (Manis javanica) phylogenetically related to SARS-CoV-2 by 85-92%, but their similarity was especially high in the RBD domain of glycoprotein S, including the six characteristic amino acids of that zone in SARS-Cov-2 [30]. According to Zhou et al. the most probable explanation of its origin would be a genetic recombination between a bat strain and that of another animal species as an intermediate host, probably a pangolin [24]. This would reinforce the idea that the optimization of protein S to bind the human ACE2 receptor is the result of natural selection and not of genetic engineering or successive passages of the virus in a laboratory. However, no virus has been obtained in the pangolin with sufficient homology to be considered the ultimate intermediary.
In the absence of a better explanation, the official theory is that SARS-VOC-2 leaped into the human from the bat by ingesting an infected animal at the Huanan (Wuhan) seafood market in late December 2019. No However, it does not seem likely that there was a direct passage from the bat to the human being for various reasons of physical impossibility:
i. Bats hibernate in natural or artificial cavities, which remain at a constant temperature and high humidity. In winter (November-March) they enter a state of lethargy, drastically decreasing their body temperature, heart and respiratory rates, reducing their metabolic activity and energy consumption to a minimum.
ii. The Malaysian pangolin, in which viruses with a compatible S protein were found, is an animal that lives in tropical areas and is not naturally present in the north-central area of China.
iii. According to Chinese epidemiologists, in this market, bats are not sold, but fish, shellfish and some conventional mammals (pig..) and
iv. The caves where the viruses with the most similarity to SARS-VOC-2 were found were collected in the Yunnan caves almost 1500 km from Wuhan. In no natural way could these bats have reached the place where the pandemic originated [31].
II Direct Jump from the Bat to the Human before the Final Mutations
Although works such as that of Andersen et al. claim that the virus spread to a human by jumping directly from the bat found in the Yunnan caves, claiming that some wild animal traffickers show seropositivity against bat coronaviruses [32]. However, the mobility of these small local traffickers in Yunnan province is limited to the neighboring cities of Kunming, Chengdu, Nanning, Chongqing and even Hanoi in Vietnam, and almost no cases of the disease appeared in these cities. On the other hand, the possibility that the virus had managed to directly affect a human and that the genes encoding protein S would mutate once in it, giving it a high affinity for the ACE2 receptor, seems a remote possibility, as numerous virologists have declared. USA, France, Australia, Sweden, England, India and China [33-38].
Like many other viruses with the RNA genome, the mutation rate of coronaviruses is 10-nucleotide substitutions (x position and year), occurring basically in the first replicative cycles. It is therefore surprising that the study of the genome of 104 SARS-Cov-2 viruses, isolated from Wuhan patients between December 2019 and February 2020, showed a homology of 99.9% in their sequences. This data suggests that this new coronavirus originated from a single source in a very short period and was detected very early in the first days of its human spread [8, 27, 39]. Li et al. carried out genealogical genetic reconstruction studies in human genomic sequences of SARS-COV-2 seem to indicate that this virus was already present in Wuhan in early November 2019 (range between September 25 and December 19 with a 95% certainty) [39]. This would imply that whatever the mechanism by which SARS-CoV-2 originated, natural or artificial, its passage to humans occurred with almost complete security in the last months of 2019. At this point, no current theory can satisfactorily explain the origin of the SARS-VOC-2 genetic sequence and how an interspecies jump occurred that managed to infect humans so effectively that their human-human transmissibility was so high.
The Hypothesis of Natural Mutation
Many articles emphatically state that SARS-CoV-2 has a natural origin. However, their claims are based on inconclusive data and relying on a future finding of the true SARS-CoV-2 ancestor virus in some intermediate host, setting their sights mostly on the pangolin. It is possible that this finding will never occur, which considerably weakens any emphatic claim regarding this hypothetical natural origin. In the article by Andersen et al., it is stated beforehand that they rule out that it is a manipulated virus instead of basing that conclusion on the study of the facts [38]. They claim that the genetic data irrefutably shows that SARS-CoV-2 is not derived from any previously used virus skeleton (such a claim would involve knowing all the chimera formation studies conducted at the University of North Carolina and Wuhan among others)
However, they recognize that “the SARS-CoV-2 BRD is optimized to bind human ACE2 with an efficient solution different from those previously verified” and they also recognize that “although the RaTG13 virus is identical to SARS-CoV-2 in a 96%, its S protein diverges into RBD, suggesting that it may not efficiently bind human ACE2.” To explain this situation, they propose that the virus pass to the pangolin since “some pangolin coronaviruses show a strong similarity to SARS-CoV-2 in the RBD”. Lastly, they affirm that “preliminary reports estimated that the ‘ancestor’ common to SARS-CoV-2 appeared between the end of November and the beginning of December 2019, data compatible with the first retrospectively confirmed cases”. To say that there are viruses in the Pangolin with similarity in protein S to that of SARS-CoV-2 that have evolved naturally and can bind to human ACE2 is one thing, but to affirm that this optimization was achieved through a possible natural hybridization between bat and pangolin coronaviruses in winter and in a city thousands of miles from the host bat's place of origin by chance change is really a risky claim, but to say its natural origin is “irrefutable” based on data so lazy it just doesn't fit reality.
If we continue analysing the arguments of the publication by Andersen et al. we see important weaknesses. The coronavirus S protein is divided into two functional units, S1 and S2. S1 facilitates virus infection by binding to host receptors. It comprises two domains, the N-terminal domain and the C-terminal RBD domain that interacts directly with host receptors. According to Andersen et al., “SARS-CoV-2 appears to be optimized to bind to the human ACE2 receptor by inserting 12 nucleotides into protein S, specifically at the border of S1 and S2 [38]. The receptor binding domain (RBD) in protein S is the most variable part of the coronavirus. Six residues in said RBD are essential for binding to the human ACE2 receptor. In SARS-CoV-2 these 6 residues are L455, F486, Q493, S494, N501 and Y505, five are mutated in SARS-CoV-2 with respect to their closest relative, BatCoV-RaTG1. This mutation means that SARS-CoV-2 can bind with high affinity to ACE2 from humans, primates, ferrets, pigs and cats. However, it hardly shows an affinity for bats, rodents and civets, which practically rules them out as the last host before the jump to humans ”.
And that “Interestingly, although the key residues in the RBD of SARS-CoV-2 are theoretically suboptimal in computational predictions, in practice SARS-CoV-2 binds with a high affinity for human ACE2, which would imply that a new optimal joining solution.” They also comment that “the second notable feature of SARS-CoV-2 is a predictive polybasic cleavage site (RRAR) at the leading protein at the junction of S1 and S2, the two subunits of the S protein. At the cleavage site two basic arginines are inserted, one alanine and one proline. This suggests that the sharp turn created by proline insertion will result in the addition of O-linked glycans to S673, T678, and S686 flanking the polybasic cleavage site. A polybasic cleavage site has never been observed in betacoronaviruses before, making it a unique feature of SARS-CoV-2.”
Although Andersen et al. affirm that these characteristics are strong evidence that SARS-CoV-2 is not the product of genetic engineering [38]. Other important virologists think exactly the opposite and that probably these differences, difficult to explain, are precisely those that seem to rule out a natural origin. It is important to note that although very similar, the BatCoV-RaTG1 differs from the SARS-CoV-2 by at least 1,100 bases [40]. This volume is a “genetic abyss” to be explained by a simple viral mutation. Since the start of the pandemic, up to 700 SARS-CoV-2 mutations have been sequenced, but in all cases they were subtle genetic differences, mere “typographical errors” and small deletions that until now have not had any functional impact [40-42]. The theory of the isolated mutation as the origin of SARS-CoV-2 has been discarded by most world researchers.
Paraskevis et al., analysed the possible genetic evolution of SARS-CoV-2 and although its genome is closely related to the BatCoV RaTG13 sequence (96.3%), it shows a discordant grouping with the bat-SARS-like coronavirus sequences that they make up an unusual middle segment never seen before in any coronavirus, which would indicate that it is a new type of coronavirus [32]. Specifically, in region 5 encompassing the first 11,498 nucleotides and the last part of region 3 comprised between positions 24,341 and 30,696, SARS-CoV-2, RaTG13 and Bat-SARS formed a single group while in the middle region spanning the 3 End of ORF1a, ORF1b and almost half of the S protein regions, SARS-CoV-2 and RaTG13 are presented as a separate distant lineage within the Sarbecovirus branch.
Their study rejects the hypothesis of SARS-CoV-2 emergence as a result of a recent recombination event. This new coronavirus provides a new lineage for almost half of its genome, with no close genetic relationships to other viruses within the Sarbecovirus subgenus. This genomic part comprises half of the region of protein S that encodes a multifunctional protein also responsible for the entry of the virus into host cells [43-45]. The most widely accepted official theory is that the Bat CoV RaTG1 virus from the Yunnan cave bat infected another animal (final host) that possessed a coronavirus capable of binding to human ACE2 receptors (such as pangolin). Both viruses infected the same cell and spontaneously recombined so that the resulting hybrid virus gained the ability to bind ACE2 and thus infect humans.
Two different mechanisms can generate virus recombinant RNA genomes [46]. 1) The rearrangement (which only occurs in segmented RNA viruses and coronaviruses are not) and 2) The recombination that is common to practically all RNA viruses. Viral recombination occurs when viruses from two different parental strains coinfect the same host cell, exchanging discrete RNA segments during replication to generate viral progeny that have some genes from both parents [47]. So if SARS-CoV-2 binds with high affinity to human ACE2 through the emergence of a new optimal binding solution (not present in other coronaviruses) and the polybasic cleavage site is a unique feature of SARS-CoV- 2, the theory that another coronavirus with ACE2 binding capacity spontaneously yielded that capacity to SARS-CoV-2 would be false (since in that case it would be monobasic).
Along these same lines, the team at Flinders University in Adelaide and Latrobe University in Melbourne carried out a study using coupling algorithms and in silico structural modeling, using the available genomic and structural biology data, to generate relevant ACE2 structural models. and use molecular dynamics to calculate the binding energies [35]. Remarkably, this approach surprisingly revealed that the binding energy between SARS-CoV-2 protein S and human ACE2 was higher than for all tested species, including the bat, the postulated source of the virus. This suggests that the SARS-CoV-2 protein S has uniquely evolved to bind to and infect cells expressing human ACE2. This finding is particularly surprising since, typically, a virus would be expected to have the highest affinity for the receptor in its original host species (bat), with a lower initial binding affinity for the receptor of any new host (human). However, in this case, the affinity of SARS-CoV-2 is higher for humans than for the host species, bats, or any other potential intermediate host species studied. Based on these data, the authors do not rule out that SARS-CoV-2 was created by a recombination event that occurred inadvertently or consciously in a laboratory.
The Virus Escape Hypothesis
I Would It be Possible for a Virus to Escape from the High Security Virological Laboratory in Wuhan?
In the first place, we should know if this center investigated this type of virus, about the creation of chimeras and the enhancement of capacities, including the jump between species. The Wuhan BSL-4 level laboratory has been studying bat coronaviruses for almost a decade under the guidance of virologist Zhengli Shi. Since 2010, his team has made at least 5 forays into the Yunnan caves to collect thousands of bats and try to detect new coronaviruses, to study their natural reservoirs, the different ways they bind to the cells it infects, see how these receptors are involved in the interspecies jump and the creation of chimeras with characteristics of different viruses.
Dr. Shi, completed part of her training as a virologist at the University of North Carolina (USA) where since 2008 various articles were published on the creation of a coronavirus chimeras replacing the receptor-binding-domain (RBD) of the S protein of the Bat-SCoV virus (bat) by that of the SARS-CoV (Human) [17, 48-52]. The Bat-SBRD mutant was able to infect mice and human cell cultures and another article on the creation of another chimera, this time using the SZ16 civet virus, which introduced the K479N mutation into the RBD of the protein. S to bind to human respiratory and brain cells [48]. Furthermore, when the viruses were inactivated with human monoclonal antibodies, the icSZ16-SK479N strain was 8 times more resistant than the rest of the mutants.
In 2010, Wuhan's lab published a study on the efficacy of ACE2 molecules from seven additional bat species studying which had the highest affinity for human SARS-CoV protein S using both the HIV-based pseudo type and the analysis of live SARS-CoV infection [12]. Then they “altered several key residues to decrease or improve the efficiency of the bat ACE2 receptor.” The conclusion of the study is that bats M. daubentoni and R. sinicus are susceptible to SARS-CoV and may be candidates as the natural host for SARS-CoV parent viruses.
Three years later, Wuhan's lab published several studies, providing the strongest evidence to date that Chinese horseshoe bats are natural reservoirs of SARS-CoV, and that intermediate hosts may not be necessary to direct human infection by some SL-CoV of bats [12-15]. SARS-CoV has been shown to use the human ACE2 molecule as its input receptor, and this is considered a hallmark of its interspecies transmissibility [15-17]. The receptor binding domain (RBD) located in the amino terminal region (amino acids 318-510) of the SARS-CoV (S) peak protein is directly involved in binding to ACE2 [16-19, 27]. However, despite the phylogenetic evidence that SARS-CoV evolved from bat SL-CoV, all previously identified SL-CoV have large SARS-CoV sequence differences in the RBD of their S protein, including one or two deletions [17-19]. “Replacing the RBD of SL-CoV protein S with that of SARS-CoV conferred the ability to use human ACE2 and replicate efficiently in mice” [27]. However, to date, SL-CoV have not been isolated from bats, and no naturally occurring SL-CoV from bats have been shown to use ACE2.
A cooperative study conducted by the University of North Carolina, led by Dr. Ralph Berrie, together with the Wuhan laboratory, describes “the creation, by the reverse genetics system, of a chimeric virus that expresses the protein S of the bat coronavirus SHC014 in a mouse-adapted SARS-CoV spine [17].” These artificial chimeras can efficiently use multiple orthologs of human ACE2, replicate efficiently in primary human airway cells, and achieve in vitro titers equivalent to the epidemic SARS-CoV strains. He further states that “in vivo experiments demonstrate the replication of the chimeric virus in the mouse lung with remarkable pathogenesis. Assessment of SARS-based immunotherapeutic and prophylactic modalities revealed poor efficacy; both the monoclonal antibody and vaccine approaches failed to neutralize and protect against SARS-CoV infection using the new spike protein. Based on these findings, we have synthetically derived a full-length infectious SHC014 recombinant virus and demonstrated robust viral replication both in vitro and in vivo.”
It is evident, when reading the text in italics, that in Wuhan bat coronaviruses were genetically manipulated with special relevance towards the creation of artificial mutations that allowed the jump between species. They describe that their self-replicating chimera can cause high-mortality pneumonia in mice and that the next step will be a study in primates. Investigations that increase virulence, ease of propagation, and hop between species are known as “gain of function”. On October 16, 2014, the Obama administration through the American government's National Institute of Health imposed a moratorium on federal funding for such research on viruses that cause SARS, influenza, and MERS known as the “Statement of funding pause on certain Types of gain-of-function research.”
However, the studies that were already underway did not stop and in 2015, a letter was published in Nature warning about “whether the artificial creation, in engineering laboratories such as Wuhan's, of viruses with possible pandemic potential justifies the risks” [53]. Many virologists question whether the information obtained from the Wuhan experiment justifies the potential risk. Richard Ebright, molecular biologist and biodefense expert at Rutgers University in Piscataway, NY (USA), believes that “The only impact of this work is the creation, in a laboratory, of a new unnatural risk” and what is needed a full scientific capacity discussion on “whether this type of chimeric virus study warrants further research versus the inherent risks involved” [54].
Along the same lines, Simon Wain-Hobson, a virologist at the Pasteur Institute in Paris, pointed out that researchers have created a new virus that “grows remarkably well” in human cells, which implies that “If the virus escaped, nobody could predict the trajectory [55].” Despite this, research in Wuhan did not stop as evidenced by the invitation made in 2018 by the Chinese Academy of Sciences to Dr. Shi Zhengli to give a lecture entitled, “Studies on bat coronavirus and its cross-species infections.” Very recently, after a multicenter study that included the Wuhan laboratory, Zhou et al. described a new bat coronavirus, which they labeled as RmYN02, from 227 Yuanan bats collected between May and October 2019; just before the first cases of SARS-CoV-2 infections appeared [56].
This new virus is more related to SARS-CoV-2 in some parts of the genome, including the longest section called 1ab in which they share 97.2% of the RNA. The authors claim that, like SARS-CoV-2, RmYN02 has amino acid insertions at the point where the two S protein subunits meet. However, they do acknowledge that the insertion of four amino acids at that point in protein S is unique to SARS-CoV-2 and has been present in all samples sequenced so far. It would seem possible that after the identification of this new RmYN02 virus, an accidental or natural recombination of this virus and RatTG13 had taken place, giving rise to a chimera that could be very similar to SARS-CoV-2.
II The Prodigious Protein S of SARS-CoV-2
i La Furina
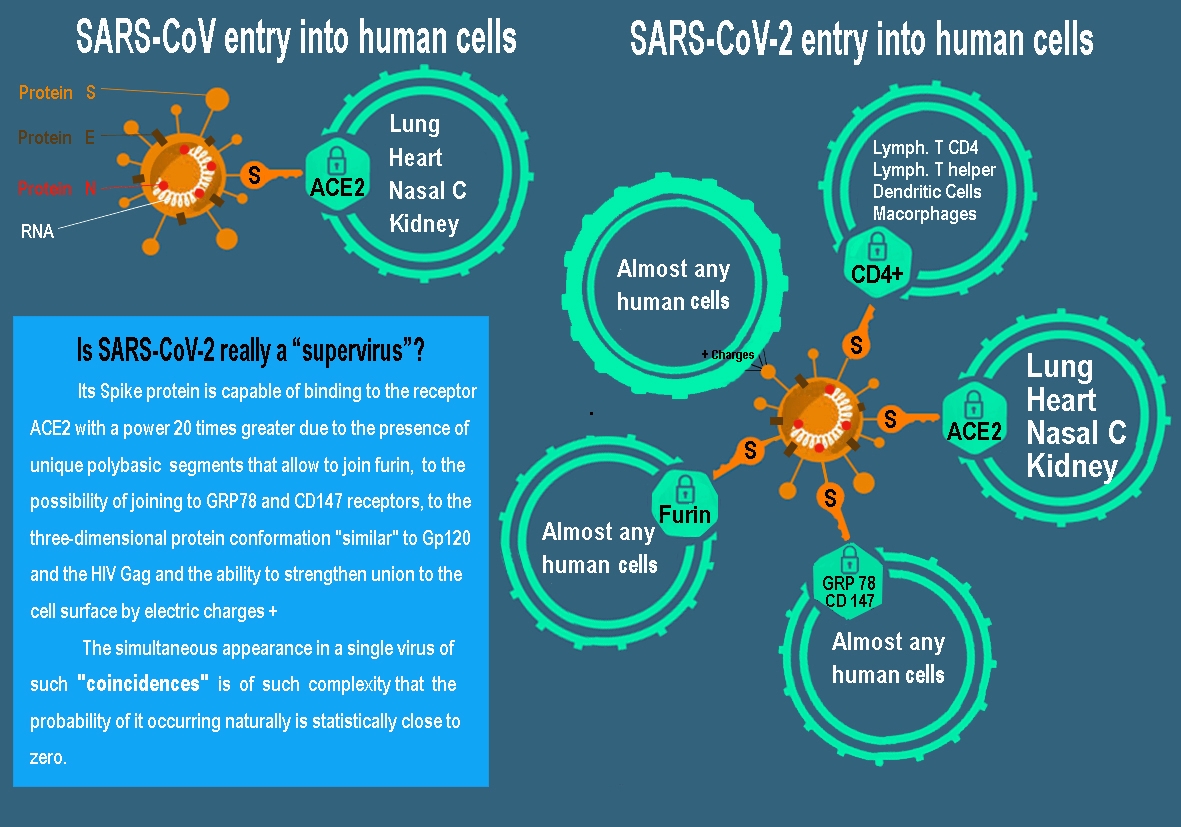
Basically, the ACE2 receptor is the human cell lock used by both SARS-CoV and SARS-CoV-2, although the latter does it up to 20 times more efficiently. However, there is another much more decisive factor to explain its ability to infect almost all body tissues: furin [57, 58]. The S1 fraction of the SARS-CoV-2 protein binds to the ACE2 receptor of the human cell, but this binding is not sufficient to achieve invasion. The S2 fraction is responsible for the fusion of the virus membrane with that of the cell. Viruses need to cut protein S to activate it and start the attack. After the fusion of its membranes, the virus introduces its genome into the cell and the process of self-replication.
The SARS-CoV used two enzymatic “scissors”, the TMPRSS2 enzymes and the cathepsins, but these are only present in some cells. In contrast, SARS-CoV-2 expresses in its protein S, 12 extra letters (ccu cgg cgg gca) that constitute a cut-off point for a third scissors: furin. This makes a great evolutionary difference, since furin is present in almost all human cells, which would explain its high transmissibility and virulence. Furthermore, furin makes a first cut of the spike of the new viruses, which already leave the human cell ‘preactivated’, allowing protein S to initiate the fusion of an infected cell with a healthy one, making the virus move freely without exposing yourself to antibodies from outside allowing it to multiply very high and spread the infection.
Figure 1: Comparison of the cellular entrance doors of the current SARS-CoV-2 and the old SARS-CoV.
Some human coronaviruses, such as HKU1, HCoV-OC43, MERS-CoV and MHV-A59, harbor multibasic cleavage sites in their S protein that allow for furin cleavage [59]. However, this cut-off point is almost non-existent in other coronaviruses and totally absent in those of the Beta group, since the S1/S2 binding site in SARS-S or RATG13-S is monobasic. Therefore, SARS-CoV-2 would be, to date, the only beta-coronavirus with this multibasic cut-off point. Indeed, bat and pangolin coronaviruses do not possess the cleavage site for furin in protein S [60, 61]. Where does that mutation come from? In theory, it is possible that certain mutation, insertion and deletion phenomena have occurred naturally in protein S in some other animal with a human-like ACE2 receptor with which another genetic recombination would have occurred in the spontaneous creation of SARS- CoV-2 with another virus that does express a multibasic area frequently, such as avian influenza virus (hemagglutinin) [56, 62, 63].
Viruses with a monobasic cleavage site are activated by TMPRSS2 or related proteases with an expression profile limited to the aerodigestive tract. As a consequence, viral replication is limited to these organs and does not cause serious disease. In contrast, viruses with a multibasic cleavage site are activated by proprotein convertases expressed throughout the body, including furin, and therefore can spread systemically and cause massive disease.
According to the French virologist Etienne Decroly of the Aix University (Marseille), it is thought that “this 12-letter insertion allows the virus to enter a greater variety of cells, which favors the spread of the virus and is key to the development of the disease” [58].
ii The GRP78 and CD147 Proteins
A Cairo University study evaluated the binding of protein S to the cell surface receptor GRP78 (Glucose Regulated Protein 78) by combined coupling of molecular modeling and structural bioinformatics [64]. They observed that the most favorable binding occurs in region IV (C480-488), calculating a binding affinity of -9.8 kcal/mol. Two other similar studies carried out in China, Germany and France support these results (in press) [65]. In this case, it is easier to explain the origin of this gain of function because GRP78 can interact with the S proteins of 2 Betacoronaviruses (MERS-CoV and bCoV-HKU9), but that would imply the participation of the genes of these virus in the creation of SARS-CoV-2 [65].
In parallel, researchers from Xian University (China) have discovered that SARS-CoV-2 can bind and penetrate a cell using the CD147 protein, an immunoglobulin that is expressed on the cell surface [66]. The genetic origin of this property is also easy to trace since it is presented by the old SARS-CoV, although it has also been detected in red blood cells and that plasmodium malaria uses to bind to them. A genomic researcher and virologist at the Wyss Institute at Harvard University, commented anonymously, that when researchers studied the entry mode of the ACE-2 receptor, it was discovered that this new coronavirus could use not only the protease called TMPRSS2 enters cells but at least 8 other different proteases, making things very difficult when trying to develop inhibitors and medications. Indeed, the numerous pathways to the cell for SARS-CoV-2 have made it known to some researchers as the supervirus.
III The Strange Presence of Gp120 and Gag Proteins in SARS-CoV-2
Protein S is divided into two subunits (S1 and S2). The S1 subunit aids receptor binding, and the S2 subunit facilitates membrane fusion [2]. Pradham et al., when studying the generic sequence that encodes this SARS-CoV-2 protein, found 4 insertions (short sequences) that they understood were unique to it and are not present in other coronaviruses and that were translated into amino acid sequences [34]. identical or very similar to those of the HIV Gp120 and Gag proteins. It is imperative to understand that although the inserts are discontinuous in the primary amino acid sequence, the 3D modeling of the final SARS protein S-protein fragments converge to constitute the receptor binding site in a way that appears functionally like those of HIV.
The first 3 identical/similar inserts were at positions 404-409, 462-467, 136-150 of the linear protein S protein molecules and the fourth at positions 366-384. As the authors affirm, these inserts are placed in an apparently random way, in the primary sequence of the SARS-CoV-2 protein S. However, during its folding to configure the tertiary protein, the first 3 inserts approach, creating a structure similar to the Gp120 protein that plays a crucial role in the recognition of the host cell by the HIV virus by binding to the primary receptor. CD4 and for its cellular penetration. But even more surprising is that said spatial coincidence occurs in a configuration closely linked to the place where the protein S that the host receptor recognizes to bind to it [67].
The 4th insert creates a protein segment like the HIV Gag, which is involved in the binding of the new virions to the host membrane, the packaging of the virus and the formation of virus-like particles [68]. Finally, the authors highlight that the 4 inserts have pI values of around 10 that can facilitate virus-host interactions. Analysis of these data leads the authors to speculate on whether these insertions provide additional flexibility to the protein S binding site by forming a hydrophilic circuit in its structure that could facilitate or improve virus-host interactions, thereby extending the range. of host cells that SARS-CoV-2 can infect. Even if such speculation is not true, it is impossible not to agree with the authors that such a coincidence is unlikely to be fortuitous in nature.
Just 4 days after the online publication of the article by Pradham et al., Xiao C et al. were quick to deny outright any possible artificial origin of SARS-CoV-2, in an article sent to be published on 4 February (curiously accepted to publish the same day ...), for this they relied on superfluous explanations that do not resolve the true significance of the findings of the University of Delhi (India) [34, 69]. Firstly, he argues that these 4 small insertions can be seen in the genome of different viruses including bacteriophages, influenza and others among which he mentions the RatTG13 coronavirus. In addition, he argues that of the three insertions that are part of a protein similar to HIV gp120, V1 is far from V4 and V5 while the one to the Gag protein is far from being part of the same structural unit. Such arguments do not contradict the initial conclusions of the study by Pradham et al., who decided to withdraw the article due to recurring pressures, pending to resend it after its review [34]. First, even accepting that there are similar inserts in other viruses, their translation into proteins does not lead to the creation of any structure that has the slightest three-dimensional relationship with the Gp120 protein that is unique and exclusive to the HIV virus.
Furthermore, they state that the computer-generated three-dimensional representation of the Gp120-like protein manufactured by SARS-CoV-2 does not exactly match that of HIV. However, they do not take into account that in cellular reality proteins combine with each other forming quaternary structures that are what really define whether the SARS-CoV-2 protein is functionally homonymous to Gp120. Finally, with respect to the last insert that would be equivalent to the Gag protein, they say that it is far from Gp120 and therefore not related to it. Interestingly, Xiao et al. forget that while Gp120 is responsible for binding to the CD4 receptor and the entry of the virus into the cell, the function of the gag protein is practically the opposite since, after infection and viral RNA translation, is responsible for recruiting two copies of the viral genomic RNA along with other cellular and viral proteins that allows the expulsion of the viral particle from the surface of an infected cell. Therefore, the fact that the Gag protein is distant from Gp120 is surely of less importance [69].
It is important to mention here that there is extensive experience in creating chimeras that include the Gp120 protein in the genome of other viruses including Ebola, Influenza, and Sendai [69-76]. The finding of genes of the HIV virus has been corroborated by two new works published on a platform of the Chinese Academy of Sciences where articles can be submitted prior to their peer review (Chinaxiv.org). According to Professor Ruan Jishou of Nankai University in Tianjin, SARS-CoV-2 has a mutated gene found in the HIV virus that is a completely unique feature among known natural coronaviruses [77]. Another similar study, carried out by the Huazong University of Science and Technology, was published in the same repository by Professor Li Hua confirming Professor Rouen's findings [78]. This study further confirms the results of Dr. Pradham stating that this HIV-like gene has not been detected in either the former SARS-CoV, MERS or Bat-CoVRaTG13.
In summary, the quaternary conformation of the SARS-CoV-2 protein generated with these inserts could function similarly to Gp120 allowing SARS-CoV-2 to bind to the cellular CD4 receptor allowing its entry into T lymphocytes, monocytes and macrophages and inducing apoptosis in them. As Warren et al. state, only experimental virology provides a way to identify animal viruses with the potential to replicate in humans because this information may not be evident from the isolated viral sequence [79].
IV Dual Binding to Membranes Using ACE Receptors and Positive Charges
Researchers from the company Immunor AS of Norway and the University of St. George of London, proposed that the method of action of the protein S effect was twofold since it included membrane components other than the ACE2 receptor that would explain its high infectivity and pathogenicity [37]. It is shocking that the authors directly affirm that it is a chimeric virus giving it as a certainty according to their observations. Simply explained, SARS-CoV-2 can bind more strongly to the ACE2 receptor due to the reinforcement that is achieved with positive electrical charges (a fact that had already been mentioned in the work of Pradham et al., According to Sorensen et al., receptor binding domain dependent phagocytosis is specifically related to the cumulative loading of sections artificially inserted into the SARS-CoV-2 protein S [34, 37]. Positively charged basic amino acid substitutions allow the formation of saline bridges with the CLEC4M/DC-SIGNR receptor or indirectly, by the additional salt bridges formed between positively charged amino acids and negatively charged phospholipids in the cell membrane.
The technology of positively charged amino acid insertion into peptides and proteins to improve cell affinity and transport of them through the cell wall has been known for more than a decade [80-82]. Sorensen et al. state unequivocally that SARS-CoV-2 uses the structure of SARS-CoV as the backbone and then creates a chimera by adding RBM 437-508 [36, 49]. But then, four of the six new loaded inserts (1, 2, 3 and 6) outside of the RBM were also excluded from its chimera, and the Cov-2-specific Cys538-Cys590 bridge that brings an additional load (pI = 10.03) right next to the RBM. Coutard et al., highlights that the enriched basic load associated with this cleavage site is found in a series of viruses, such as HIV, influenza, Cytomegalovirus (herpes), respiratory syncytial virus, Zika and Ebola viruses [58].
The clinical evidence of this pandemic suggests that SARS-CoV-2 presents an extended cellular tropism that confers neurological, hematological and immunological pathogenicity that cannot be explained by the ACE2 receptor alone. The mechanism of action linked to basic Arginine-rich domains is known as “Binding of peptides that penetrate cells” [82]. The important point to understand is that such positively charged amino acids must be located in such a way that they cover four amino acids (or more) in length so that they act as an initial membrane anchor allowing their binding and direct cellular absorption depending on the peptide’s net charge [80].
Additional collateral support for this hypothesis is the findings of Zhou et al. who in 2018 isolated a new coronavirus that they called SADS (Acute Porcine Diarrhea Syndrome) [25]. Despite painstaking studies, they were unable to find evidence of involvement of any of the three known receptors from previous SARS epidemics: ACE2, Aminopeptidase N and Dipeptidyl peptidase 4. Both SADS and SARS-CoV-2 appeared to have an accessory/different and more promiscuous co-receptor like CLEC4M/DC-SIGN driven by a positively charged S-trimer surface. Based on these converging data, they state that the general method of action for SARS-CoV-2 is, as a co-receptor dependent phagocytic process. The virus binds to ACE2 receptors in its receptor binding domain, but in addition positive charges stabilize said binding by the electrical attraction - of opposite charge - of cell membrane co-receptors such as CLEC4M/DC-SIGNR and possibly to the membrane itself whose charge is globally negative.
Again, an article proposing an artificial origin of SARS-CoV-2, was immediately vilified, this time by “Fact Check” companies that criticized a possible conflict of interest because they are manufacturing a vaccine. Obviously, such an accusation is false because this fact is recognized from the beginning by the authors and because there is no clash of interests between creating a vaccine and claiming that the origin of the virus is artificial. Likewise, these journalists converted to scientists, affirm that the theory of the electrical union of positively charged amino acids to the cell surface is not proven when in fact it is a technique used in the laboratory for more than a decade.
According to the US Center for Disease Control and Prevention (CDC), dozens of accidents, of lesser or greater severity, occur every year in microbiological experimentation laboratories around the world, including the USA, where there is a detailed record of them. Rather, China does not publicly disclose such information. A scientific article published in 2018 has already revealed widespread systemic deficiencies in these centers [83, 84].
V Promiscuity of SARS-CoV-2 Protein S in Its Binding to Cells
Most patients with severe COVID-19 develop acute respiratory distress syndrome (ARDS), a clinical phenomenon marked by the development of bilateral infiltrates and hypoxemia, defined as a decrease in the ratio of arterial PO2 to inhaled FiO2. Many of them will require mechanical ventilation and most of them will die. However, COVID-19 is not just a lung disease. The cells that express ACE2 are expressed in many tissues of the body with special concentration, it is expressed abundantly in the alveolar lung cells, the mucosa of the small intestine and the renal tubular cells, but it is also significantly expressed in the cells of the vascular endothelium, mesenchymal cells and the smooth muscle of virtually all organs.
This ubiquity, associated with a binding capacity 20 times greater than that of SARS-CoV, explains why COVID-19 can affect any tissue in the body including other of the aforementioned to the heart, esophagus, ileum, kidneys, bladder, bile duct, liver, testicles, olfactory bulb and CNS [2, 85-88]. We now know that SARS-CoV-2 can bind extremely effectively with the ACE2 receptor due to the special conformation of its extraordinary S protein due to the presence of reinforcements for binding to the cell surface through its exclusive use of alternative entry mechanisms (furina, GRP78, polybasic cleavage site, CD47, CD 147, Gp120 and positive charges). Undoubtedly, a greater spectrum of receptors to which to couple and greater effectiveness/binding strength allows it to penetrate practically any cell line in the body.
SARS-CoV-2 produces serious tissue injuries due to the sum of its action mechanisms. Firstly, lung involvement produces generalized hypoxia phenomena that are aggravated by systemic involvement of the endothelium of blood vessels that generates the formation of micro and macrothrombi that aggravate ischaemia and cause death of anoxic tissues [87-89]. These thrombotic manifestations include pulmonary embolisms, deep vein thrombosis, catheters, and even arterial thrombosis. In addition, microvascular thrombosis, acrosyndrome and capillary leak syndrome also occur, affecting the lungs, kidneys, and heart, which may ultimately lead to organ failure and death [90-93].
But in addition, the ease of binding of SARS-CoV-2 to the CD4 receptors of the cells of our immune system produces a reduction of the immune cells, mainly CD3 +, CD4 +, CD8 + NK and CTL lymphocytes, which are associated with a worse prognosis of the disease. All this can be associated with a reduced expression of IFN-γ by the auxiliary T cells or an expanded population of circulating monocytes that secrete IL-6 and IL-1β that will end up generating a storm of cytokines that create an environment of hyperimmunity. For this reason, in many cases a Th1/Th2 imbalance occurs that generates autoimmune-type reactions and aggravates all tissue injuries in the body [94-99]. Regardless of whether innate immune system-mediated toxic inflammation contributes to COVID-19-related morbidity and mortality, it is clear that viral spread is a key factor in severe disease.
Detection of circulating viral RNA in peripheral blood is strongly related to the severity of the disease. Similarly, necropsy or liver and renal biopsy studies in infected patients have revealed the presence of inclusion bodies almost universally, secondary to viral persistence in the tissues [89-93]. This high replication capacity is another of the characteristics of SARS-CoV-2, probably in relation to the presence of the furin cleavage site and the Gag molecule, which are also related to the packaging of new virions or their transmission directly. from cell to cell without exposing ourselves to our immune system [34, 58-60, 76, 77]. All of these findings greatly complicate the chances that SARS-CoV-2 is a natural recombination of the genome of only 2 viruses that co-infected the same cell. There are no known two viruses that can fulfill this theory, even assuming subsequent mutations, couplings or deletions of their genome. Even imagining a very high mutational rate, it would not be reasonable for this to be the way of creating the virus.
VI Summary of the Data Examined
The various genetic peculiarities discovered in SARS-CoV-2 can be explained naturally. However, as the number of abnormalities causing some gain of function increases (such as better binding to the cells it infects and broadening the spectrum of cells it is able to penetrate), the statistical chances of such an event occurring decrease. randomly in nature.
i. The SARS-CoV-2 virus has only recently appeared, and its evolution/transformation includes rare/absent characteristics in betacoronaviruses.
ii. The SARS-CoV-2 protein S binds to the ACE2 receptor with an efficiency 20 times superior to that of the previous SARS-CoV, being even higher compared to other coronaviruses
iii. Although SARS-CoV-2 shares most of its genome with BatCoV RaTG13 and RmYN02 bat coronaviruses but they differ widely in their S protein
iv. Although SARS-CoV-2 and some pangolin coronaviruses have great similarity in their S protein but much lower in the rest of the genome.
v. The SARS-CoV-2 protein S can be cut with the TMPRSS2 enzyme and cathepsins, which are present in some cell types.
vi. The SARS-CoV-2 protein S expresses 12 extra letters (ccu cgg cgg gca) that allow the furin cut that is present in almost all human cells, which could explain its high transmissibility and virulence.
vii. Only the HKU1, HCoV-OC43, MERS-CoV and MHV-A59 coronaviruses harbor multibasic cleavage sites in their S protein that allow for furin cleavage. This characteristic is totally absent in beta-coronaviruses (with monobasic binding sites). SARS-CoV-2 is the only known beta-coronavirus with this multibasic cutoff point.
viii. The SARS CoV-2 protein S can bind to GRP78, something that only two Betacoronaviruses (MERS-CoV and bCoV-HKU9) can do, but that would involve the participation of the genes of these viruses in the creation of SARS-CoV-2.
ix. SARS CoV-2 protein S can bind and penetrate a cell using CD147 protein just like the old SARS-CoV did
x. The SARS-CoV-2 protein S surface expresses 3 inserts that generate a segment like HIV Gp120 that would allow it to bind to the CD4 receptor of cells of the immune system
xi. The SARS-CoV-2 protein S expresses on the surface 1 insert that generates a segment similar to the HIV Gag
xii. The SARS-CoV-2 can bind more strongly to the ACE2 receptor due to the reinforcement achieved with additional positive electrical charges (pI = 10.03) right next to the RBM
xiii. During its evolution, SARS-CoV-2 has acquired so many novel features that it provides a gain-of-function in such a short period of time that the possibility that it had been created through reorganization, mutations, re-coupling or deletion processes is statistically tiny.
xiv. In Wuhan, coronavirus chimeras have been created for 10 years, so thinking about a possible accidental escape is statistically more likely than the theoretical infection of a cell by two or more viruses simultaneously to create a new virus by recombining its genetic materials. infectivity + pathogenicity surpasses all existing coronaviruses.
Conclusion
It should be completely clear that the possibility of a bacteriological warfare or a deliberate release of SARS-CoV-2 by China has never been considered in this article, it is only analysed if the accidental departure of a chimera coronavirus that was experienced in Wuhan's microbiological laboratory is a plausible theory. No conclusion will be drawn from the content of this article, it has only been attempted to expose the existing data on the subject but analysing it under a new perspective that differs in many aspects from the official explanation. Now, it is the reader who must assess the data provided and draw their own conclusions.
Addendum
Regardless of the opinion of each reader, the lesson that remains is that virological experimentation of the “gain-of-function” type of aspects as critical as the jump from animals to humans should be immediately prohibited worldwide or, at the less, strictly controlled by international organizations under pain of serious economic sanctions to countries that fail to comply. If not, even if this global pandemic was not caused by an artificial virus, the next one will be and its consequences even more incalculable.
Acknowledgements
My special recognition towards the Spanish researcher A.E.L. and the Indian virologist A.A.M. They have helped me understand the real meaning of published data on the possible origin of SARS-CoV-2 but prefer that their names be listed anonymously.
Article Info
Article Type
Review ArticlePublication history
Received: Mon 29, Jun 2020Accepted: Thu 09, Jul 2020
Published: Fri 17, Jul 2020
Copyright
© 2023 Alejandro Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2020.01.06
Author Info
Corresponding Author
Alejandro SousaRegional Hospital of Monforte, Lugo, Spain
Figures & Tables
References
- GenBank (2020) Wuhan seafood market pneumonia virus isolate Wuhan-Hu-1, complete genoma. GenBank MN 908947.
- J Reina (2020) El SARS-CoV-2, una nueva zoonosis pandémica que amenaza al mundo. Vacunas 21: 17-22.
- Zhang T, Wu Q, Zhang Z (2020) Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Cell Biol 30: 1346.e2-1351.e2. [Crossref]
- Xu X, Cheu P, Wang J, Feng J, Zhou H et al. (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63: 457-460. [Crossref]
- Khailanya R, Safdarb M, Ozaslanc M (2020) Genomic characterization of a novel SARS-CoV-2. Gene Rep 19: 100682. [Crossref]
- Ji W, Wang W, Zhao X, Zai J, Li X (2020) Homologous recombination within the spike glycoprotein of the newly identified coronavirus 2019-nCoV may boost cross-species transmission from snake to human. J Med Virol.
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E (2020) A novel coronavirus emerging in China. Key questions for impact assessment. N Engl J Med 382: 692-694. [Crossref]
- Lu R, Zhao X, Li J, Niu P, Yang B et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565-575. [Crossref]
- Cui J, Han N, Streicker D, Li G, Tang X et al. (2007) Evolutionary relationships between bat coronaviruses and their hosts. Emerg Infect Dis 13: 1526-1532. [Crossref]
- Shi Z, Hu Z (2008) A review of studies on animal reservoirs of the SARS coronavirus. Virus Res 133: 74-87. [Crossref]
- Bai B, Hu Q, Hu H, Zhou P, Shi Z et al. (2008) Virus-Like Particles of SARS-Like Coronavirus Formed by Membrane Proteins from Different Origins Demonstrate Stimulating Activity in Human Dendritic Cells. Plos One 3: e2685. [Crossref]
- Hou Y, Peng C, Yu M, Li Y, Han Z et al. (2010) Angiotensin-converting enzyme 2 (ACE2) proteins of different bat species confer variable susceptibility to SARS-CoV entry. Arch Virol 155: 1563-1569. [Crossref]
- Ren W, Li W, Yu M, Hao P, Zhang Y et al. (2010) Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J Gen Virol 87: 3355-3359. [Crossref]
- Yuan J, Hon C, Li Y, Wang D, Xu G et al. (2010) Intraspecies diversity of SARS-like coronaviruses in Rhinolophus sinicus and its implications for the origin of SARS coronaviruses in humans. J Gen Virol 91: 1058-1062. [Crossref]
- Yu M, Chameri G, Zhi Z, Wang L (2010) Identification of key amino acid residues required for horseshoe bat angiotensin-I converting enzyme 2 to function as a receptor for severe acute respiratory syndrome coronavirus. J gen Virol 91 :1708-1712. [Crossref]
- Ge X, Li H, Yang X, Chmura A, Zhu G et al. (2013) solation and Characterization of a Bat SARS-like Coronavirus That Uses the ACE2 Receptor. Nature 503: 535-538. [Crossref]
- Menachery V, Yount B, Debbink K, Agnihothram S, Gralinski LE et al. (2015) A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21: 1508-1513. [Crossref]
- Yang Y, Liu C, Du L, Jiang S, Shi Z et al. (2015) Two Mutations Were Critical for Bat-to-Human Transmission of Middle East Respiratory Syndrome Coronavirus. J Virol 89: 9119-9123. [Crossref]
- Zeng L, Gao Y, Ge X, Zhang Q, Peng C et al. (2016) Bat Severe Acute Respiratory Syndrome-Like Coronavirus WIV1 Encodes an Extra Accessory Protein, ORFX, Involved in Modulation of the Host Immune Response. J Virol 90: 6573-6582. [Crossref]
- Ge X, Wang N, Zhang W, Hu B, Li B et al. (2016) Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol Sin 31: 31-40. [Crossref]
- Zeng L, Ge X, Peng C, Tai W, Jiang S et al. (2017) Cross-neutralization of SARS coronavirus-specific antibodies against bat SARS-like coronaviruses. Sci China Life Sci 60: 1399-1402. [Crossref]
- Hu B, Zeng L, Yang X, Ge X, Zhang W et al. (2017) Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog 13: e1006698. [Crossref]
- Zhou P, Fan H, Lan T, Yang X, Shi W et al. (2018) Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 556: 255-258. [Crossref]
- Peng Zhou, Xing-Lou Yang, Xian-Guang Wang, Ben Hu, Lei Zhang et al. (2020) A neumonía outbreak associated with a coronavirus of probable bat origin. Nature 579 :270-273. [Crossref]
- Luo C, Wang N, Yang X, Liu H, Zhang W et al. (2018) Discovery of Novel Bat Coronaviruses in South China That Use the Same Receptor as Middle East Respiratory Syndrome Coronavirus. J Virol 92: e00116- e00118. [Crossref]
- Li B, Si H, Zhu Y, Yang X, Anderson D et al. (2019) Discovery of Bat Coronaviruses through Surveillance and Probe Capture-Based Next-Generation Sequencing. mSphere 5: e00807- e00819. [Crossref]
- Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 94: e00127- e00120. [Crossref]
- Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181-192. [Crossref]
- Hadfield J, Megill C, Bell S, Huddleston J, Potter B et al. (2018) Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 34: 4121-4123. [Crossref]
- Lam TTY, Shum MH, Zhu HC, Tong YG, Ni XB et al. (2020) Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L et al. (2020) Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv.
- Andersen KG, Rambault A, Lipkin WI et al. (2020) The Proximal Origin of SARS-CoV-2. Nat Med 26: 450-452. [Crossref]
- Paraskevis D, Kostaki E, Magiorkinis G, Panayiotakopoulos G, Sourvinos G et al. (2020) Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol 79: 104212. [Crossref]
- Pradhan P, Kumar A, Mishra A, Gupta P, Kumar P et al. (2020) Uncanny similarity of unique inserts in the 2019-nCoV spike protein to HIV-1 gp120 and Gag.
- Xiao B, Xiao L (2020) The possible origins of 2019-nCoV coronavirus.
- Piplani S, Kumar P, Winkler D, Petrovsky N (2020) In silico comparison of spike protein-ACE2 binding affinities across species; significance for the possible origin of the SARS-CoV-2 virus. arXiv 2005: 06199.
- Sorensen A, Susrud A, Dalgleish AG (2020) A Candidate Vaccine for Covid-19 (SARS-CoV-2) Developed from Analysis of its General Method of Action for Infectivity. QRB Discovery (Cambridge Coronavirus Collection).
- Montagnier L (2020) https://www.infobae.com/america/mundo/2020/04/27/el-virologo-que-gano-el-premio-nobel-por-descubrir-el-vih-aseguro-que-el-nuevo-coronavirus-fue-creado-en-un-laboratorio/.
- Li X, Zai J, Wang X, Li Y (2020) Potential of large “first generation” human-to-human transmission of 2019-nCoV. J Med Virol 92: 448-454. [Crossref]
- Foster P, Forster L, Renfrew C, Foster M (2020) Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A 117: 9241-9243. [Crossref]
- Rambaut A (2020) Phylogenetic analysis of nCoV-2019 genomes.
- Laamarti M, Alouane T, Kartti S, Elfihri C, Hakmi M et al. (2020) Large scale genomic analysis 1 of 3067 SARS2 CoV-2 genomes reveals a clonal geo-distribution 3 and a rich genetic variations of hotspots mutations. bioRxiv.
- Zhang Y, Holmes E (2020) A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell 181: 223-227. [Crossref]
- Gregory J Babcock, Diana J Esshaki, William D Thomas Jr, Donna M Ambrosino (2004) Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol 78: 4552-4560. [Crossref]
- Li W (2005) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24: 1634-1643. [Crossref]
- Sanjuan R, Domingo P (2016) Mechanisms of viral mutation. Cell Mol Life Sci 73: 4433-4448. [Crossref]
- Perez M, Arenas M, Galan JC, Palero F, Gonzalez F (2015) Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect Genet Evol 30: 296-307. [Crossref]
- Becker M, Graham RL, Donaldson EF, Rockx B, Sims AC et al. (2018) Synthetic Recombinant Bat SARS-like Coronavirus Is Infectious in Cultured Cells and in Mice. Proc Natl Acad Sci U S A 105: 19944-19949. [Crossref]
- Scobey T, Yount BL, Sims AC, Donaldson EF, Agnihoehram SS et al. (2013) Reverse Genetics With a Full-Length Infectious cDNA of the Middle East Respiratory Syndrome Coronavirus. Proc Natl Acad Sci U S A 110: 16157-161562. [Crossref]
- Sheaham T, Rockx B, Donaldson E, Sims A, Pickles R et al. (2008) Mechanisms of Zoonotic Severe Acute Respiratory Syndrome Coronavirus Host Range Expansion in Human Airway Epithelium. J Virol 82: 2274-2285. [Crossref]
- Rockx B, Sheaham T, Donaldson E, Harkema J, Sims A et al. (2007) Synthetic Reconstruction of Zoonotic and Early Human Severe Acute Respiratory Syndrome Coronavirus Isolates That Produce Fatal Disease in Aged Mice. J Virol 81: 7410-7423. [Crossref]
- Sheaham T, Rockx B, Donaldson E, Corti D, Baric C (2008) Pathways of Cross-Species Transmission of Synthetically Reconstructed Zoonotic Severe Acute Respiratory Syndrome Coronavirus. J Virol 82: 8721-8732. [Crossref]
- Butler D (2015) Engineeered bat virus stirs debate over risky research. Nature.
- https://www.eurekalert.org/pub_releases/2013-10/ea-nsc103013.php
- https://www.eleconomista.es/internacional/noticias/10545707/05/20/Las-quimeras-del-instituto-de-virologia-de-Wuhan-el-epicentro-del-covid19.html
- Zhou H, Chen C, Hu T, Li J, Song H et al. (2020) A novel bat coronavirus reveals natural insertions at the S1/S2 cleavage site of the Spike protein and a possible recombinant origin of HCoV-19.
- Markus Hoffmann, Hannah Kleine-Weber, Stefan Pöhlmann (2020) A Multibasic Cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Molecular Cell 78: 779-784. [Crossref]
- B Coutard, C Valle, X de Lamballerie, B Canard, N G Seidah et al. (2020) The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 176: 104742. [Crossref]
- Yang Yang, Lanying Du, Chang Liu, Lili Wang, Cuiqing Ma et al. (2014) Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 111: 12516-12521. [Crossref]
- Tommy Tsan-Yuk Lam, Na Jia, Ya-Wei Zhang, Marcus Ho-Hin Shum, Jia-Fu Jiang et al. (2020) Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 583: 282-285. [Crossref]
- Xingguang Li, Junjie Zai, Qiang Zhao, Qing Nie, Yi Li et al. (2020) Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 92: 602-611. [Crossref]
- Tao Zhang, Qunfu Wu, Zhigang Zhang (2020) Probable Pangolin Origin of SARSCoV-2 Associated with the COVID-19 Outbreak. Curr Biol 30: 1346.e2-1351.e2. [Crossref]
- Jasmina M Luczo, John Stambas, Peter A Durr, Wojtek P Michalski, John Bingham (2015) Molecular pathogenesis of H5 highly pathogenic avian influenza: the role of the haemagglutinin cleavage site motif. Rev Med Virol 25: 406-430. [Crossref]
- Ibrahim M Ibrahim, Doaa H Abdelmalek, Mohammed E Elshahat, Abdo A Elfiky (2020) COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect 80: 554-562. [Crossref]
- Hin Chu, Che-Man Chan, Xi Zhang, Yixin Wang, Shuofeng Yuan et al. (2018) Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J Biol Chem 293: 11709-11726. [Crossref]
- Wang K, Chen W, Zhou Y, Lian J, Zhang Z et al. (2020) SARS-CoV-2 invades host cells via a novel route: CD147-spike protein.
- Robert N Kirchdoerfer, Christopher A Cottrell, Nianshuang Wang, Jesper Pallesen, Hadi M Yassine et al. (2016) Pre-fusion structure of a human coronavirus spike protein. Nature 531: 118-121. [Crossref]
- Daniel R Beniac, Anton Andonov, Elsie Grudeski, Tim F Booth (2006) Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol 13: 751-752. [Crossref]
- Chuan Xiao, Xiaojun Li, Shuying Liu, Yongming Sang, Shou-Jiang Gao et al. (2020) HIV-1 did not contribute to the 2019-nCoV genome. Emerg Microbes Infect 9: 378-381. [Crossref]
- D Yu, T Shioda, A Kato, M K Hasan, Y Sakai et al. (1997) Sendai Virus-Based Expression of HIV-1 gp120: Reinforcement by the V(-) Version. Genes Cells 2: 457-466. [Crossref]
- S Li, V Polonis, H Isobe, H Zaghouani, R Guinea et al. (1993) Chimeric influenza virus induces neutralizing antibodies and cytotoxic T cell against human immunodeficiency virus type. J Virol 67: 6659-6666. [Crossref]
- Aneesh Vijayan, Juan García-Arriaza, Suresh C Raman, José Javier Conesa, Francisco Javier Chichón et al. (2015) A Chimeric HIV-1 gp120 Fused with Vaccinia Virus 14K (A27) Protein as an HIV Immunogen. Plos One 10: e0133595. [Crossref]
- X Pang, M Zhang, A I Dayton (2001) Development of dengue virus replicons expressing HIV-1 gp120 and other heterologous genes: a potential future tool for dual vaccination against dengue virus and HIV. BMC Microbiology 1: 28. [Crossref]
- K M Copeland, A J Elliot, R S Daniels (2005) Functional Chimeras of Human Immunodeficiency Virus Type 1 gp120 and Influenza A Virus (H3) Hemagglutinin. J Virol 79: 6459-6471. [Crossref]
- Xiaozhuo Ran, Zhujun Ao, Adriana Trajtman, Wayne Xu, Gary Kobinger et al. (2017) HIV-1 envelope glycoprotein stimulates viral transcription and increases the infectivity of the progeny virus through the manipulation of cellular machinery. Scientific Rep 7: 9487. [Crossref]
- Francesca Beneduce, Yuri Kusov, Matthias Klinger, Verena Gauss-Müller, Graziella Morace (2002) Chimeric hepatitis A virus particles presenting a foreign epitope (HIV gp41) at their surface. Antiviral Res 55: 369-377. [Crossref]
- Ruan Jishou et al. (2020); https://www.chinaxiv.org/abs/202002.00082.
- Li Hua et al. (2020) https://www.chinaxiv.org/abs/202002.00062.
- Cody J Warren, Sara L Sawyer (2016) How Host Genetics Dictates Successful Viral Zoonosis. PloS Biol 17: e3000217. [Crossref]
- Helene L Amand, Carolina L Boström, Per Lincoln, Bengt Nordén, Elin K Esbjörner (2011) Binding of cell-penetrating penetratin peptides to plasma membrane vesicles correlates directly with cellular uptake. Biochim Biophys Acta 1808: 1860-1867. [Crossref]
- Jean Philippe Richard, Kamran Melikov, Eric Vives, Corinne Ramos, Birgit Verbeure et al. (2003) Cell- penetrating Peptides: A re-evaluation of the mechanism of cellular uptake. J Biol Chem 278: 585-590. [Crossref]
- P E Thorén, D Persson, M Karlsson, B Nordén (2000) The Antennapedia peptide penetratin translocates across lipid bilayers – the first direct observation. FEBS letters 482: 265-268. [Crossref]
- A Peters (2018) The global proliferation of high-containment biological laboratories: understanding the phenomenon and its implications. Rev Sci Tech 37: 857-883. [Crossref]
- Michael J Selgelid (2016) Gain-of-Function Research: Ethical Analysis. Sci Eng Ethics 22: 923-964. [Crossref]
- Fan Wu, Su Zhao, Bin Yu, Yan-Mei Chen, Wen Wang et al. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579: 265-269. [Crossref]
- Chaolin Huang, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506. [Crossref]
- Yingjie Zhu, Ziqiang Du, Yanfang Zhu, Wenfeng Li, Hongjun Miao et al. (2020) Evaluación de la función del órgano en pacientes con infección grave por COVID-19. Med Clin. [Crossref]
- Yuhao Zhang, Xiuchao Geng, Yanli Tan, Qiang Li, Can Xu (2020) New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmater 127: 110195. [Crossref]
- Guang Chen, Di Wu, Wei Guo, Yong Cao, Da Huang et al. (2020) Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest 130: 2620-2629. [Crossref]
- Jun Jie Ng. (2020) Thromboembolic events in patients with SARS-CoV-2. J Vasc Surg. [Crossref]
- J M Barrios-López, I Rego-García, C Muñoz Martínez, J C Romero-Fábrega, M Rivero Rodríguez et al. (2020) Ischaemic stroke and SARS-CoV-2 infection: a causal or incidental association? Neurología 35: 295-302. [Crossref]
- Huan Han, Lan Yang, Rui Liu, Fang Liu, Kai-Lang Wu et al. (2020) Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 58: 1116-1120. [Crossref]
- Bérangère S Joly, Virginie Siguret, Agnès Veyradier (2020) Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med 1-4. [Crossref]
- L He, Y Ding, Q Zhang, X Che, Y He et al. (2006) Expression of elevated levels of pro‐inflammatory cytokines in SARS‐CoV‐infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 210: 299-297. [Crossref]
- Vardhana A, Wolchok J (2020) The many faces of the anti-COVID immune response. J Exp Med 217: e20200678. [Crossref]
- Alba Grifoni, Daniela Weiskopf, Sydney I Ramirez, Jose Mateus, Jennifer M Dan et al. (2020) Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181: 1489-1501.e15. [Crossref]
- J M Urra, C M Cabrera, L Porras, I Ródenas (2020) Selective CD8 cell reduction by SARS-Cov2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol 217: 108486 [Crossref]
- Rui Liu, Ying Wang, Jie Li, Huan Han, Zunen Xia et al. (2020) Decreased T cell populations contribute to the increased severity of COVID-19. Clin Chim Acta 508: 110-114 [Crossref]
- Leila Mousavizadeh, Sorayya Ghasemi (2020) Genotype and phenotype of COVID-19: Their roles in pathogenesis.
J Microbiol Immunol Infect. [Crossref]

