Sentinel Lymph Nodes Detection in Early-Stage Breast Cancer: ICG-Enhanced Fluorescence and Radioactivity Detection Methods Comparison
A B S T R A C T
Introduction: A sentinel lymph node biopsy (SLNB) is a standard procedure for surgical staging in the early stage of breast cancer, avoiding excess lymphadenectomy in most patients. Among the new methods of SLNB, there is a prospective method based on the use of indocyanine green (ICG) as a contrast medium. Numerous studies to assess the possibility of the routine use of ICG fluorescence method generally favored the use of ICG. The main issue discussed is whether it can be used alone or in combination with the RI method. In this work, using the specified device, we conducted a study aimed at intercomparing of ICG fluorescence imaging and the radioactivity detection methods in order to assess prospects for the use of the ICG method of imaging for SLN detection in early-stage breast cancer.
Material and Methods: A prospective, non-randomized single-center study was conducted at the oncological breast unit Pavlov State Medical University, Russia. The study included 32 patients aged from 34 to 78 (median-55.2 years) with breast cancer (cTis-3, N0-2). 4 patients underwent surgery after neoadjuvant systemic treatment. 30-45 minutes before the surgery, additionally, 2 ml of the ICG solution was administered near the tumor margin via single skin puncture. The solution was prepared by dissolving 25 mg ICG in 20% of human albumin. Then the place of injection was massaged for at least 5 minutes. SLN biopsy was performed by two criteria by the presence of radioactivity in the axillary region, which was monitored using a handheld gamma-detector or by the ICG fluorescence, location of which was visualized using the ICG-Scope system. The lymph node was recognized as a sentinel if its intensity exceeded the background radioactivity level of 99mTc or exceeded the threshold value upon the ICG fluorescence, which was 1% of the standard sample intensity.
Keywords
Breast cancer, signal, sentinel lymph node, indocyanine green, NIR fluorescence
Introduction
In modern breast cancer surgery, it is important to reduce the frequency of axillary lymphadenectomies due to the high traumatism of this procedure, which does not increase overall survival [4]. A sentinel lymph node biopsy (SLNB) is a standard procedure for surgical staging in the early-stage of breast cancer, avoiding excess lymphadenectomy in most patients [5]. Herewith, a comparison of the long-term outcomes (ALMANAC studies) of lymph node dissection and sentinel node biopsy demonstrate that the relative risks of upper limb lymphedema within 12 months after the axillary lymphadenectomy are approximately 13% versus 5% after SLNB. Also, nerve injury with loss of upper limb sensitivity amounts to 31% after lymphadenectomy, while the incidence of this complication after SLNB is no more than 11%. The period of drainage use, duration of hospitalization and time to return to conventional limb activity were also statistically lower in the SLNB group [6].
For the first time, isosulfan blue was proposed for sentinel (signal) lymph nodes (SLN) detection in 1994. Later, in addition to blue dyes for these purposes, the Technetium-99m labeled (99mTc) radiopharmaceutical began to be widely used both as a standalone, and in combination (99mTc + blue). The advantage of the blue dye is the accessibility and lack of need for special equipment, since their location can be determined on visual inspection. However, such simplicity of detection leads to the identification of SLNs located only on the wound surface. In addition, the use of the blue dye in high concentrations (which is inevitable for imaging with common (non-fluorescent) dyes may lead to adverse reactions, including skin necrosis, indurations with accompanying pain, up to anaphylaxis and pulmonary edema [7]. Since the idea of SLNB is to remove all potentially dangerous lymph nodes including those with metastases, we should consider high requirements to the sensitivity to the methods of their detection. Although radioisotope (RI) method is one of the most commonly used methods, nowadays, the double contrast study (99mTc+blue) is considered to be the "gold standard” in breast cancer with clinically negative lymph nodes [2]. Moreover, the best mapping results are achieved with periareolar subdermal dye administration. Among the new methods of SLNB, there is a prospective method based on the use of indocyanine green (ICG) as a contrast medium [1]. ICG, which is used in humans for over 50 years, is a fluorescent dye. Its uptake is detected by imaging systems operating in the near infrared region (NIR) of the spectrum [8, 9].
The first multicenter study on the applicability of ICG fluorescence for SLN detection in breast cancer, conducted in the USA with the use of Mini-FLARETM system for NIR imaging, established the optimal ICG concentration within 0.4-0.8 mM (0.3-0.6 mg/ml). At the same time, SLN identification was successful in 94 out of 95 patients (99%) using NIR imaging only or in combination with the radioisotope method. 100% of 177 resected SLN (mean 1.9, range of 1-9) showed NIR fluoresence and only 88% had radioactivity. In 2 of 95 patients (2.1%), SLNs contained gross-metastases, which were found only with NIR fluorescence, and in one of the patients this led to the upstaging to N1 IIb [10]. Numerous studies to assess the possibility of the routine use of ICG fluorescence method generally favored the use of ICG. The main issue discussed is whether it can be used alone or in combination with the RI method [3]. In addition to the mentioned device (Mini-FLARE™), there are different other systems used for NIR fluorescence imaging: Photo DynamicEye (PDE; Hamamatsu Photonics, Japan), IC-view, ICG-F, Hiper-Eye, Stortz Vitom II ICG/NIR.
In our previous studies, we used the ICG-Scope NIR imaging system developed in the Russian Scientific Center in Seoul. It was used to determine infarction areas, to evaluate the lymphatic system status, for lymph ducts mapping in plastic surgery, to identify SLN in gastric cancer and lung cancer [11-15]. In this work, using the specified device, we conducted a study aimed at intercomparison of ICG fluorescence imaging and the radioactivity detection methods in order to assess prospects for the use of the ICG method of imaging for SLN detection in early-stage breast cancer.
Material and Methods
A prospective, non-randomized single-center study was conducted at the 5th Oncology Department (Mammology) of Pavlov First Saint Petersburg State Medical University with the approval from the ethics committee of the institution. It should be noted that we observed all principles of the Declaration of Helsinki 2013. Before enrollment, we obtained written informed consent from patients. The study included 32 patients aged from 34 to 78 (median-55.2 years) with breast cancer (cTis-3, N0-2). 4 patients underwent surgery after neoadjuvant systemic treatment. At the time of a surgery the clinical lymph node status in all patients was N0 with T≤2 (Table 1).
Table 1: Patients characteristic.
|
Criterion |
Number of patients |
% of total |
W/o changes post-surgery, number of patients |
% of all patients with this criterion |
W/ changed criterion post-surgery, number of patients |
% of all patients with this criterion |
Comments |
|
Tis |
1 |
3.1 |
1 |
100 |
- |
- |
|
|
Т1 |
19 |
59.4 |
18 |
94.7 |
1 |
5.3 |
Changed to Т0 (post neoadjuvant polychemotherapy) |
|
Т2 |
11 |
34.4 |
7 |
63.6 |
4 |
36.4 |
Tumor regression at the background of neoadjuvant polychemotherapy in 1 patient, criterion pT became less than cT upon the pathomorphological diagnosis in other 3 patients. |
|
Т3 |
1 |
3.1 |
- |
- |
1 |
100 |
Reduction to T at the background of neoadjuvant polychemotherapy |
|
N0 |
32 |
100 |
28 |
87.5 |
4 |
12.5 |
tumor regression to T1N0M0 in the patient with cТ3N2aМ0 at the background of neoadjuvant polychemotherapy, but the pathomorphological investigation revealed N2а l/nodes status. |
12-24 hours before the surgery, peritumoral injection of 99mTc (V.G. Khlopin Radium Institute, St. Petersburg) at a dose of 150-200 MBq. 30- 45 minutes before the surgery, additionally, 2 ml of the ICG solution was administered near the tumor margin via single skin puncture. The solution was prepared by dissolving 25 mg ICG (Pulsion Medical Systems AG, Germany) in 20% of human albumin. Then the place of injection was massaged for at least 5 minutes.
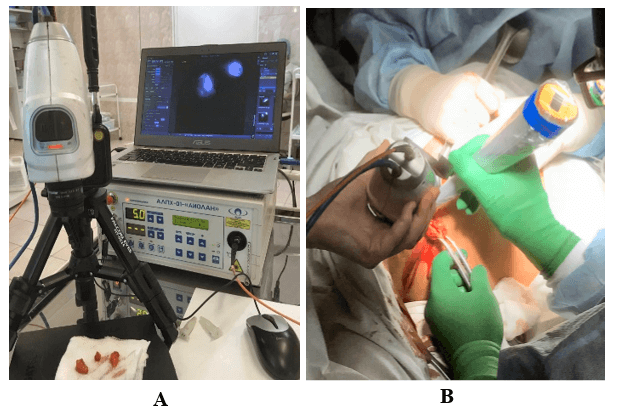
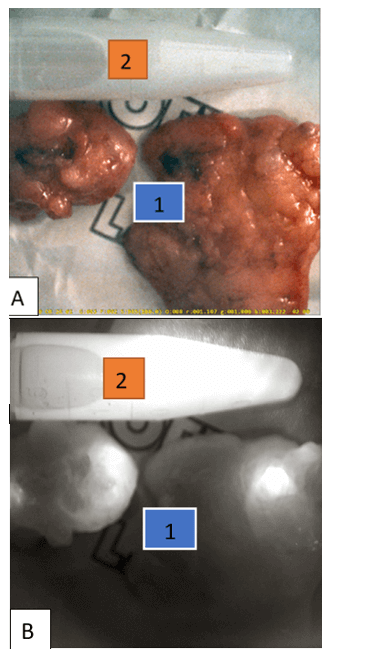
SLN biopsy was performed by two criteria: by the presence of radioactivity in the axillary region, which was monitored using a handheld gamma-detector (Gamma Finder, Germany) or by the ICG fluorescence, location of which was visualized using the ICG-Scope system (Russia-Republic of Korea) (Figure 1). The system provides the ability to visualize a 43x43 mm field from a distance of 210 mm, both in white light and in NIR fluorescence light with laser excitation at 808 nm. It was used intraoperatively and in the postoperative period, which made it possible to detect SLN, as well as perform their quantitative assessment by the ICG fluorescence. In the latter instance, the camera head was mounted on a tripod providing a fixed distance to the preparations, and the system was calibrated using a standard sample fluorescent in the NIR (Figure 2). The lymph node was recognized as a sentinel if its intensity exceeded the background radioactivity level of 99mTc or exceeded the threshold value upon the ICG fluorescence, which was 1% of the standard sample intensity.
Figure 1: ICG-Scope system including NIR-camera, laser apparatus and a laptop. A) on the “back-table” when examining the resected SLNs, B) during the intraoperative search for the SLNs using the NIR camera and gamma probe simultaneously.
Outcomes
The study was conducted from September 2019 to February 2020. At the initial stage of the surgical intervention immediately after the identification of SLN location in the axillary region by one of the applied methods (Figure 2B), the biopsy was performed. Resected material was placed on a table with the ICG-Scope (back-table) system, with the help of which the ICG and 99mTc intensity were estimated (Figure 2A). SLN was not found only in one of 32 patients using all testing methods. Thus, the sensitivity of both methods turned out to be the same-97%. However, they differed significantly in terms of the number of identified SLNs. The total number of nodes detected using ICG only was 107 (average value per one patient 3.3, range 1-8), and using only 99mTc-77 (2.4, 1-7). Double labeling did not reveal additional nodes, i.e. all SLNs detected with 99mTc were identified with ICG. Histological investigations showed that 9 SLNs in 6 (19%) patients had metastases, where 2 SLNs (1 patient) were detected only with ICG. No side effects were registered post ICG administration.
Figure 2: Two resected SLNs (1) and the standard sample (2): А) in the white light, B) in the NIR fluorescence light.
The use of the sentinel lymph node detection using isotope scanning simplifies the intraoperative search: monitoring with the Gamma-Finder sensor helps to sufficiently determine the location and direction of manipulations to detect the lymph node before surgery. Whereas the use of the ICG method reliably reveals a node only if its visualization is not obstructed by the adipose tissue in the axillary region. At the same time, the fluorescence method seems to be more sensitive: in a number of surgeries, some nodes identified with ICG were not observed with the use of isotopic method only. Another peculiarity is that the ICG method may confuse the surgeon with insufficient experience: often the lymphatic ducts filled with the drug can be mistaken with contrast-filled lymph nodes. It is necessary to follow their course (without damage) in the cellular tissue of the axillary region with extreme care up to the lymph node determined by palpation or visually. This tactic allows us to detect lymph nodes under the adipose tissue, which are undetected during the primary examination of the operating field. The need for accuracy of manipulations when tracing the course of the lymphatic ducts is due to the safety issue. In this case, the fluorescent fluid flows into the wound, and makes it difficult to find lymph nodes using the ICG method.
The use of ICG is attractive in comparison with radioactive testing because it helps to avoid several restrictions, such as the need for a radiologic license, the need to prepare, use and dispose of the radiopharmaceutical, and the patient's non-consent for its injection. In addition, mapping with ICG has a number of added benefits possibility to administer the drug directly within the operating room, when the patient is already under anaesthesia; possibility to carry out a targeted and constant visualization of the anatomical structures of the lymphatic system with the help of NIR camera, as well as the lack of necessity in special devices for drug handling, and lower cost, which can also be reduced by using an ICG + human albumin mixture.
Discussion
SLN detection method performance evaluation is usually based on two indicators: 1) sensitivity, which is defined as the proportion of patients with at least one SLN detected (detection rate, SLNs is successfully identified); and 2) mean number of detected SLNs among all examined patients [2, 3].
The sensitivity in this study was 97% (SLN was not identified only in one of 32 patients). The same result was recently noted in a study in China (200 patients), comparing ICG, blue dye and (ICG+blue dye)-based methods, and amounted to 97, 89 and 99.5%, respectively [16]. Sensitivity is a rather crude marker, since it does not take into account the total number of detected nodes. For example, in this study, the RI method revealed only one SLN in every of 4 cases, while the ICG method revealed 4 times more in the same patients.
Therefore, when comparing methods, it is reasonable to use the mean number of detected nodes per patient. In our case, this indicator was 2.4 for 99mTc and 3.3 for ICG (1.39 times higher). The advantage of ICG over 99mTc was also shown during the recent randomized study performed in France, where the average number of biopsies in the group with 99mTc composed 1.77, and 2.14 (1.2 times higher) with double detection (ICG + 99mTc) [17]. Comparison with a wider range of data that we performed on the basis of reviews and (21 studies, from 2009 to 2015), supplemented by current data, showed 99mTc 1.35-2.3 (1.8), blue 1-2.4 (1.8), ICG 1.75-5.4 (2.7), 99mTc + blue 1.5-2.4 (1.9), (99mTc + ICC) 1.5-2.4 (1.9) (in brackets - the mean value for all published studies) [2, 3].
Mean number of lymph nodes that we detected using the technetium isotope almost coincides with the published data (2.4 vs 2.3), which indicates the absence of problems with the RI technique, including the used domestic preparation. However, it is known that it is impossible to identify all SLNs using the RI method, as evidenced by the fact of wide use of combined test (99mTc+blue dye) instead of the use of blue dye and 99mTc separately. Despite the same detecting performance, their combined use allows us to improve the specific detection rate (at the average of 1.8 to 1.9).
As for NIR imaging, according to the study results, the number of identified SLNs using ICG was much higher than with blue dye or 99mTc [2, 3]. The greater sensitivity of ICG compared to the blue dye scan is explained by: 1) higher sensitivity of fluorescence methods compared to absorption methods, which is widely used in analytic chemistry to detect substances in minor concentrations, 2) ICG operation in the NIR spectroscopy, allowing visualization of SLNs at a depth of up to 10 mm, while blue dye can only detect the ones on the surface. Samorani et al.
prospectively compared the number of resected lymph nodes labeled with the use of ICG and 99mTc and obtained an average of 2 in both groups, suggesting that to enhance the stained lymph nodes, it was necessary to increase the concentration of administered ICG [18]. In our study, the concentration was 0.5 mg/ml. From our perspective, it is inappropriate or even harmful to raise it above the specified value due to the so-called reabsorption phenomenon [12]. This is particularly confirmed by the fact that the lowest detection rate of 1.75 was obtained in a study, when significantly higher concentration of ICG was used [19]. At the same time, an increase in concentration to 5 mg/ml is justified in the absence of a NIR camera, since it allows, as with blue dye, to identify nodes with an unassisted eye [20]. For the achievement of maximum success, it is proposed to administer 99mTc in combination with ICG [21].
Other researchers understand this as a waste of resources and time of a patient. They believe that ICG imaging can be used as one single method for proper and accurate identification of sentinel lymph nodes without additional resources associated with the administration of 99mTc and without side effects associated with the use of blue dyes [22, 23]. Our study supports the last point of view, as all SLNs detected with 99mTc have been identified with ICG, and adding the radioactive method would only complicate the procedure and raise the cost. In terms of absolute values, the detection rate was, on average, 22% higher than the published data on ICG (3.3 vs. 2.7) but lower than the maximum registered values (3.3 vs. 5.4). Should we work towards to increase this indicator? Some authors have raised fair concerns about the excessive number of staining sentinel lymph nodes when using ICG as a marker. They believe that excessive removal can lead to lymph drainage defect from the upper limb, which leads to postoperative lymphatic complications, including the risk of wound infection, lymphedema, numbness, decreased shoulder mobility, chronic pain, etc. [24]. It seems that the level we have achieved satisfies the ALARP principle, and therefore the need to remove a larger number of lymph nodes remains questionable. Additional special studies are necessary to assess that issue.
ICG-guided NIR imaging is effective, convenient and can be proposed as an equivalent replacement for the intraoperative sentinel lymph nodes identification using 99mTc. As a dye, ICG is not expensive, but a NIR camera is required. However, experience indicates that it is increasingly found in operating rooms due to the need for NIR navigation during various surgical interventions. It is known that the sentinel lymph nodes identification method using ICG has a very small number of side effects. In support of its safety, none of patients from our study experienced any adverse events. Technetium 99m is more expensive especially considering extra costs for its handling and storage. Through the use of a ICG and albumin mixture, we were able to significantly reduce the cost of the procedure, since we used only one package of ICG (25 mg) for 25 subjects. 99mTc brings additional difficulties to the patient due to the discomfort and duration of the procedure, as well as the difficulties associated with the interaction with radioactive materials.
Conclusions
ICG revealed 39% more SLNs compared to 99mTc in the early-stage breast cancer. All SLNs with radioactivity also had increased NIR fluorescence. Therefore, ICG fluorescence can be recommended as the exclusive method to solve this problem. Visualization using ICG fluorescence can be safely and effectively used for intraoperative lymphatic mapping in real time.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 05, Jun 2020Accepted: Tue 16, Jun 2020
Published: Tue 23, Jun 2020
Copyright
© 2023 Anton Telishevskiy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COCB.2020.01.07
Author Info
Anton Telishevskiy G. V. Papayan I. A. Chizh I. A. Vinogradov N. N. Petrischev V. V. Kolarkova
Corresponding Author
Anton TelishevskiySurgeon of Onсological Breast Unit, The Institute of Surgery and Emergency Medicine, Pavlov First Saint Petersburg State Medical University, Saint Petersburg, Russia
Figures & Tables
Table 1: Patients characteristic.
|
Criterion |
Number of patients |
% of total |
W/o changes post-surgery, number of patients |
% of all patients with this criterion |
W/ changed criterion post-surgery, number of patients |
% of all patients with this criterion |
Comments |
|
Tis |
1 |
3.1 |
1 |
100 |
- |
- |
|
|
Т1 |
19 |
59.4 |
18 |
94.7 |
1 |
5.3 |
Changed to Т0 (post neoadjuvant polychemotherapy) |
|
Т2 |
11 |
34.4 |
7 |
63.6 |
4 |
36.4 |
Tumor regression at the background of neoadjuvant polychemotherapy in 1 patient, criterion pT became less than cT upon the pathomorphological diagnosis in other 3 patients. |
|
Т3 |
1 |
3.1 |
- |
- |
1 |
100 |
Reduction to T at the background of neoadjuvant polychemotherapy |
|
N0 |
32 |
100 |
28 |
87.5 |
4 |
12.5 |
tumor regression to T1N0M0 in the patient with cТ3N2aМ0 at the background of neoadjuvant polychemotherapy, but the pathomorphological investigation revealed N2а l/nodes status. |
References
- Ferrucci M, Franceschini G, Douek M (2018) New techniques for sentinel node biopsy in breast cancer. Transl Cancer Res 7: S405-S417.
- Ahmed M, Purushotham AD, Douek M (2014) Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol 15: e351-e362. [Crossref]
- Sugie T, Ikeda T, Kawaguchi A, Shimizu A, Toi M (2017) Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol 22: 11-17. [Crossref]
- Rao R, Euhus D, Mayo HG, Balch C (2013) Axillary node interventions in breast cancer: a systematic review. JAMA 310: 1385-1394. [Crossref]
- Larson KE, Valente SA, Chao Tu, Dalton J, Grobmyer SR (2018) Surgeon-associated variation in breast cancer staging with sentinel node biopsy. Surgery 164: 680-686. [Crossref]
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG et al. (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst 98: 599-609. [Crossref]
- Efron P, Knudsen E, Hirshorn S, Copeland EM (2002) Anaphylactic reaction to isosulfan blue used for sentinel node biopsy: case report and literature review. Breast J 8: 396-399. [Crossref]
- Papayan GV, Akopov AL (2016) Fluorescence diagnostics in the near-IR: Apparatus, application. J Opt Technol 83: 536.
- Papayan G, Akopov A (2018) Potential of indocyanine green near-infrared fluorescence imaging in experimental and clinical practice. Photodiagnosis Photodyn Ther 24: 292-299. [Crossref]
- Martin RC, Edwards MJ, Wong SL, Tuttle TM, Carlson DJ et al. (2000) Practical Guidelines for Optimal Gamma Probe Detection of Sentinel Lymph Nodes in Breast Cancer: Results of a Multi-Institutional Study. For the University of Louisville Breast Cancer Study Group. Surgery 128: 139-144. [Crossref]
- Sonin D, Papayan G, Pochkaeva E, Chefu S, Minasian S et al. (2017) In vivo visualization and ex vivo quantification of experimental myocardial infarction by indocyanine green fluorescence imaging. Biomed Opt Express 8: 151-161. [Crossref]
- Papayan GV, Akopov AL, Antonyan PA, Llin AA (2018) Infrared fluorescence lymphography in experimental and clinical conditions. Region Circ Microcirc 17: 86-93.
- Volokh MA, Papayan GV, Akopov AL (2018) Assessment of the lymphatic system prior to and after various options for face lifting using fluorescent visualization in the near infrared range. Prob Reconstruct Plastic Surg 3: 5-11.
- Papayan G (2013) ICG Fluorescent and radioisotope methods comparison for sentinel lymph nodes detection in gastric cancer surgery. 10th international gastric cancer congres. Verona 129.
- Akopov AL, Papayan GV, Chistyakov IV, Karlson A, Gerasin AV et al. (2015) Intraoperative Detection of Sentinel Lymph Nodes Using Infrared Imaging System in Local Non-Small Cell Carcinoma of Lung. Vestn Khir Im I I Grek 174: 13-17. [Crossref]
- Guo J, Yang H, Wang S, Cao Y, Liu M et al. (2017) Comparison of sentinel lymph node biopsy guided by indocyanine green , blue dye , and their combination in breast cancer patients : a prospective cohort study. World J Surg Oncol 15: 196. [Crossref]
- Vermersch C, Raia Barjat T, Chapelle C, Lima S, Chauleur C et al. (2019) Randomized comparison between indocyanine green fluorescence plus 99m technetium and 99m technetium alone methods for sentinel lymph node biopsy in breast cancer. Sci Rep 9: 6943. [Crossref]
- Samorani D, Fogacci T, Panzini I, Frisoni G, Accardi FG et al. (2015) The use of indocyanine green to detect sentinel nodes in breast cancer: A prospective study. Eur J Surg Oncol 41: 64-70. [Crossref]
- Murawa D, Hirche C, Dresel S, Hünerbein M (2009) Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 96: 1289-1294. [Crossref]
- Yamamoto S, Maeda N, Yoshimura K, Oka M (2013) Intraoperative detection of sentinel lymph nodes in breast cancer patients using ultrasonography-guided direct indocyanine green dye-marking by real-time virtual sonography constructed with three-dimensional computed tomography-lymphography. Breast 22: 933-937. [Crossref]
- Sugie T, Kinoshita T, Masuda N, Sawada T, Yamauchi A et al. (2016) Evaluation of the Clinical Utility of the ICG Fluorescence Method Compared with the Radioisotope Method for Sentinel Lymph Node Biopsy in Breast Cancer. Ann Surg Oncol 23: 44-50. [Crossref]
- Verbeek FPR, Troyan SL, Mieog JSD, Liefers G, Moffitt LA et al. (2014) Near-infrared Fluorescence Sentinel Lymph Node Mapping in Breast Cancer: A Multicenter Experience. Breast Cancer Res Treat 143: 333-342. [Crossref]
- Zhang X, Li Y, Zhou Y, Mao F, Lin Y et al. (2016) Diagnostic performance of indocyanine green-guided sentinel lymph node biopsy in breast cancer: A meta-analysis. PLoS One 11: e0155597. [Crossref]
- Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS et al. (2006) Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol 13: 491-500. [Crossref]
