Splenectomy before Allogeneic Hematopoietic Stem-cell Transplantation for Myelofibrosis: Two Cases
A B S T R A C T
Myelofibrosis (MF) is a clonal hematological malignancy characterized by splenomegaly, systemic symptoms, myelofibrosis, and a tendency to transform into acute leukemia. MF treatment includes anemia treatment, hydroxyurea, JAK inhibitors (JAKi) allogeneic hematopoietic stem cell transplantation (allo-HSCT), among which allo-HSCT is the only possible cure for MF. The effect of splenomegaly on the prognosis of allo-HSCT with myelofibrosis and whether splenic treatment is needed before transplantation has not been determined. A recent retrospective study from the EBMT working group found that splenectomy significantly reduced transplant-related non-recurrent mortality and did not increase the risk of disease recurrence in MF patients with pre-transplant splenomegaly. Two cases of MF splenectomy combined with allo-HSCT are reported.
Keywords
Splenectomy, myelofibrosis, massive splenomegaly, allogeneic hematopoietic stem cell transplantation
Case Data
Case 1
A 64 -year-old female patient was first admitted to hospital in 2022-03-24 because she found a mass in the left upper abdomen for more than 10 years. More than 10 years ago, the patient found a mass in the left upper abdomen, and the blood routine check of the local hospital included leukocyte 20.7 × 10E9/L, haemoglobin 188g/L and platelet 27×10E9/L. Bone marrow smear showed that the proliferation of bone marrow was not active, the number of nucleated cells decreased, and the number of lobulated nuclear cells increased. Pathological examination of bone marrow biopsy: the proliferation of bone marrow hematopoietic tissue was active, the proportion of granulocyte was increased, mainly middle and late juvenile cells, the number of megakaryocytes increased significantly with fibrous tissue hyperplasia, which was consistent with bone marrow fibrosis. The immunophenotype of bone marrow showed that there was no increase of primordial cells. MPN related gene mutation JAK2V617 mutation was positive, BCR/ABLP210 (-), BCR/ABLP190 (-), BCR/ABLP230 (-). It was diagnosed as primary myelofibrosis.
After 5 years of oral administration of thalidomide, the spleen gradually enlarged. 5 years ago, the oral administration of "ruxolitinib tablet 20mg/ day" was adjusted, and the spleen gradually shrank, and the platelet count was maintained at about 20×10E9/L. Reexamination of bone marrow smear 4 months ago showed that the myeloid original cells accounted for 2.168%. Mpn-related gene mutations: 94.5% of JAK2 mutations, 1.6% of ASXL1 mutations, and 47.5% of JAK3 mutations. Continue the oral administration of ruxolitinib, gradually reduce the dose. In the process of reduction, spleen enlarged again, and platelets decreased progressively, maintaining about 10×10E9/L. She was admitted to our hospital for further treatment. Blood routine examination on admission showed leukocyte count of 8.22×10E9/L, a neutrophil count of 5.94×10E9/L, a haemoglobin count of 84g/L and a platelet count of 8×10E9/L. Upper abdominal CT scan showed giant spleen (about 180×169×247 mm). The patient was diagnosed with primary bone marrow fibrosis (PMF) had DIPSS score 4 (medium-risk -2), DIPSS-PLUS score 3 (medium-risk -2), MPN10 score 32; new scoring system, MIPSS 70 8 points (high risk); MIPSS 70+V2.0 7 points (high risk) ,G1PSS 2 points (medium risk -2).
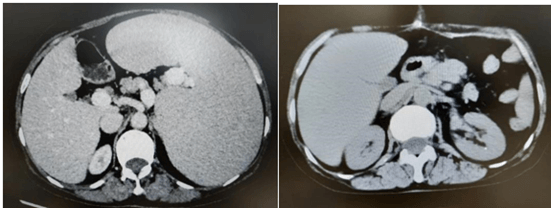
The patient had a history of primary myelofibrosis for many years, and the treatment effect of ruxolitinib was poor, the disease progressed, platelets were significantly reduced, and the spleen was progressively enlarged. Stratified according to the risk, the patient belonged to the medium-high risk group, with indications of transplantation, and the patient had a large spleen, which may affect implantation after transplantation. The preoperative platelet count was 8×10E9/L before splenectomy, which could be increased to 116×10E9/L after platelet transfusion. 2022-4-9 total splenectomy was performed, the operation was smooth, the postoperative recovery was good, no infection, bleeding and other complications occurred. Abdominal computed tomography before and after splenectomy are showed in (Figure 7). The resected spleen specimen is showed in (Figure 9A). 2022-05-21 patients were pretreated with Flu+Bu+ATG (Flu 46.7mg -d10 to -d5+Bu 42.9 mg q6h -d 6 to d-5+ATG 200mg -d4 to -d1) regimen. 2022-05-31 was infused with daughter haploidentical peripheral blood stem cells (B to AB).
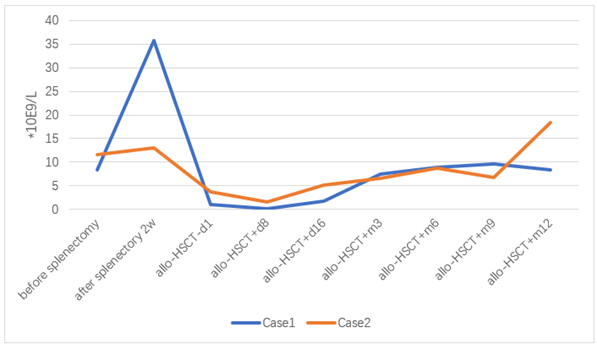
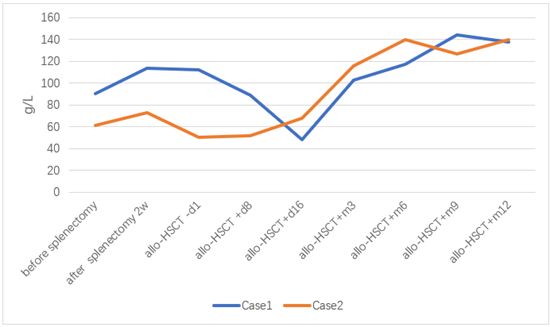
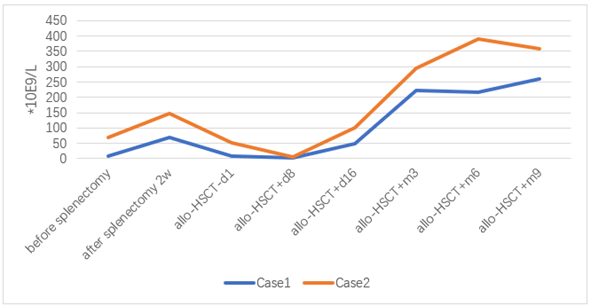
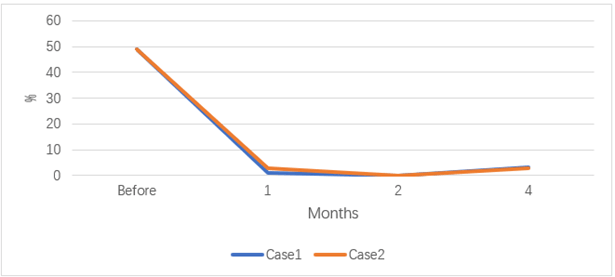
After 18 days of transplantation, ANC was greater than 0.5×10E9 / L, and after 17 days of transplantation, PLT was greater than 20×10E9/L. 2022-06-29 reexamination of bone marrow JAK2 V617F mutation 0.89%. Bone marrow flow cytometry showed no significant abnormal primitive myeloid cell population and MRD was negative. 2022-08-10 bone marrow routine review showed 60% eosinophils, BMR; MRD negative; negative for the JAK2 V617F mutation. 2022-10-12 reexamination of bone marrow routine showed that 2% of the original granulocytes; MRD negative. JAK2 V617F mutation 3.1%. STR 99%. 2022-11-09 negative for JAK2 V617F mutation. 2022-10-25 after cyclosporine reduction, the patient developed skin rash on face, neck and hands; 2022-12-07 the right shoulder joint movement is limited, the right hand lifting difficulty; 2023-01-14 mouth ulcer, eyelid congestion; GVHD was considered and improved after symptomatic treatment. At present, 1 year after transplantation, regular outpatient follow-up, the disease continued to remission. The blood cell counts before and after splenectomy and allo-HSCT are showed in (Figures 1-4). The JAK2 V617F mutation and STR data are shown in (Figures 5 & 6).
Case 2
Fang XX, female, 54 years old, was admitted to hospital on November 12, 2021, mainly due to myofibrosis, which had been diagnosed for more than 13 years. The patient was diagnosed with myofibrosis 13 years ago due to "persistent redness and fever in the head and neck" and was treated with "interferon, getriol, hydroxyurea, leucoran". Bone marrow biopsy 10 years ago showed low hyperplasia of bone marrow hematopoietic tissue, normal granuloerythroid ratio, mainly middle-to-late young cells, megakaryocytes, and moderate proliferation of reticular fibers. The bone marrow biopsy 8 years ago showed that the proliferation of bone marrow tissue was extremely active, the proportion of granulocytes was fine, erythroid cells could be seen in all stages, and most of the granulocytes were rod-divided megakaryocyte proliferation and scattered distribution. proliferation of small lymphocytes in small foci of hematopoietic tissue: reticular fibers were significantly increased with focal collagenization.
After that, the follow-up was interrupted, interferon was stopped and treated with "traditional Chinese medicine". Splenomegaly was found 4 years ago, and spleen B ultrasonography showed splenomegaly (92 mm thick, 203 mm long). Immunohistochemical classification showed that basophils accounted for 3.29% of non-erythroid cells and abnormal granulocytes accounted for 84.28% of non-erythroid cells. Chromosome 46, XX, del(3) (p11P12), de(13) (q14q22)(10). JAK2 V617F mutation 91%, BCR/ABL negative. Ruxolitinib was started four years ago. Three years ago, spleen ultrasonography showed splenomegaly (70 mm thick, 205 mm long). Anemia appeared one year ago, with low platelets, haemoglobin maintained at about 70-85g/L, and platelets at about 70-80×10E9/L. The spleen was enlarged progressively in the past 6 months, and allogeneic hematopoietic stem cell transplantation was proposed. Abdominal colour ultrasonography indicated splenomegaly (238 mm long). Bone marrow biopsy showed granulored megakaryocyte hyperplasia with partial megakaryocyte atypia, reticular fiber staining (MF-3 grade), consistent with myofibrotic changes. Chromosome: 48 XX, +9del(13) (q14q22), +mar; /49 ldem+8 [1, 2]. Myeloid hot spot mutation: JAK2 88.9%, RB1 55.99%, diagnosis of myelinosis DIPSS-plus: 4 points (high risk), MIPSS-70 score: 5 points (high risk), MIPSS-70-Plus score: 6 points (high risk); MTSS score age (57 years), platelet (<150), white blood cell (> 2.5), KPS (< 90), driver mutation (non-CALR /MPL) 2 scores, ASXL1, MMUD 2 scores; score 5 high level, transplant 5 years NRM 36%, OS 50%.
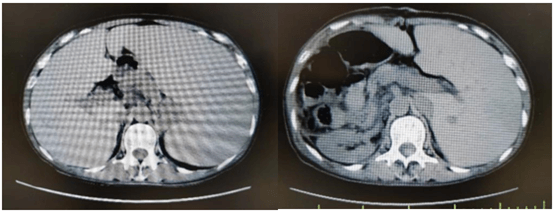
Considering the influence of spleen size on hematopoietic and cell implantation, splenectomy was performed in 2021-08-31. Abdominal computed tomography before and after splenectomy are showed in (Figure 8). The resected spleen specimen is showed in (Figure 9B). After the operation, high fever occurred, the body temperature reached 40℃, and the temperature was controlled after anti-infection treatment. 2021-10-12 Liver enhanced CT showed multiple embolism in the right anterior portal vein, main portal vein and splenic vein, and ischaemic changes in the right liver segment. The patient developed portal thrombosis. After oral administration of rivaroxaban, the portal vein ultrasound showed that the portal vein blood flow was patency. 2021-10-28 routine bone marrow examination showed no bone marrow granules, and an equal amount of nucleated cells. Bone marrow biopsy showed that hematopoietic tissue hyperplasia was generally normal (50%). Reticular fiber dyeing MF2 grade. 2021-11-13 Pre-treatment with Flu+Bu+ATG (Flu 50 mg d-10 to d-5+Bu 50 mg q6h, d-6 to d-5+ATG 200 mg d-4 to d-1) regimen, 2021-11-23 Back transfusion of nephew semi-compatible peripheral blood stem cells (AB for B, donor hepatitis B negative), transplantation +12 days, ANC greater than 0.5×10E9/L; after transplantation +13 days, platelets were greater than 20× 10E9/L (Figures 1-4). 2021-12-29 review JAK2 V617F 2.8%. 2022-02-25 bone marrow routine BMR review; MRD negative. STR 99%. 2022-03-02 test negative for JAK2 V617F. 2022-06-08 review of bone marrow routine BMR; MRD negative. Negative for JAK2 V617F (Figure 5). 2022-11-29 reexamination of bone marrow routine BMR, mild bone fiber; MRD negative. STR:BM 98.5%; T 98.1%; NK 98.8%; B 99.5% (Figure 6). 2023-02-21 routine bone marrow BMR reexamination, myofibrosis grade I. STR: BM 99%;T 98.3%. 2022-01-05 rash of both lower limbs with pruritus; 2022-02-23 two eyeballs conjunctival congestion with dryness; 2022-10-15 scattered erythema with pruritus throughout the body; GVHD was considered and improved after symptomatic treatment. At present, 18 months after transplantation, regular outpatient review, the condition has been in remission.
Figure 1: Leukocyte count before and after splenectomy and allo-HSCT.
Figure 2: Neutrophil count before and after splenectomy and allo-HSCT.
Figure 3: Haemoglobin before and after splenectomy and allo-HSCT.
Figure 4: Platelet count before and after splenectomy and allo-HSCT.
Figure 5: JAK2V617F mutation before and after transplantation.
Figure 6: STR after transplantation.
Figure 7: Case 1 before and after splenectomy.
Figure 8: Case 2 before and after splenectomy.
Figure 9: The resected spleen specimens of A) Case 1 and B) Case 2.
Discussion
In recent years, the application of allo-HSCT in the treatment of MF is gradually increasing, and we pay more and more attention to the treatment of specific clinical manifestations of MF. The treatment of splenomegaly has been a controversial issue.
In patients with MF, splenomegaly is usually secondary to extramedullary hematopoiesis, especially when visceral thrombosis is formed, portal hypertension may aggravate its development. More than 80% of MF patients showed varying degrees of splenomegaly at the time of diagnosis. Some studies have shown that splenomegaly is one of the risk factors for poor prognosis of allo-HSCT in myelofibrosis. In the early stage after allo-HSCT, the enlarged spleen can gather CD34+ hematopoietic stem cells and delay their homing to the bone marrow; after hematopoietic stem cells homing, the enlarged spleen may increase the aggregation and destruction of newly generated normal peripheral blood cells, resulting in delayed recovery of blood cell count, especially delayed implantation of neutrophils and platelets, increasing the risk of infection and bleeding. In addition, some studies have suggested that splenomegaly will increase hepatic blood flow, resulting in increased portal pressure, leading to early liver dysfunction in allo-HSCT [3-5].
Considering the adverse effects of splenomegaly on the allo-HSCT outcome of myelofibrosis, splenectomy before transplantation can promote faster implantation of hematopoietic stem cells and reduce non-recurrent mortality, but splenectomy has significant risks, including perioperative complications, operation-related death and liver function damage [6]. Some studies have reported that the incidence of perioperative complications of splenectomy before transplantation is 38%, the postoperative bleeding rate is 14%, the postoperative infection rate is 20%, and the operative mortality is as high as 18% [7]. Wong et al. studied the changes of liver function in patients with MF within 6 weeks after allo-HSCT [8]. The incidence of severe hyperbilirubinemia and sinusoidal obstruction syndrome in the splenectomy group was significantly higher than that in the control group.
Other studies have suggested that splenectomy may increase the risk of recurrence after transplantation. Spleen plays a key role in the occurrence and development of MF disease. In some cases, additional cytogenetic and molecular abnormalities that do not exist in bone marrow can be detected in the resected spleen, confirming the leukemia transformation caused by splenic hematopoiesis [9, 10]. Splenectomy may increase the circulation of bone marrow fibrotic cells with higher genetic complexity and homing in the bone marrow [9]. In 1998, an Italian team reported through a tendency score analysis that the risk of mother cell transformation after splenectomy was more than tripled [11].
In recent years, there are many contradictory reports about the effect of splenectomy before allo-HSCT on the prognosis of myelofibrosis, but there is no clear consensus. Recently, EBMT has published a related paper, which focuses on the definition, prevalence and significance of splenomegaly before allo-HSCT [12]. The latest medical management of splenomegaly (such as JAKi, routine treatment and research drugs), and alternatives in the event of failure (such as splenectomy, splenic irradiation and partial splenic embolization).
A retrospective study from the CMWP of the EBMT confirmed the clinical significance of splenomegaly before allo-HSCT. Of approximately 2000 patients with myelofibrosis who underwent allo-HSCT across Europe, 401 of 546 (73%) had an enlarged spleen more than 5 cm below the LCM at the time of transplantation, and 135 (25%) had an enlarged spleen exceeding 15 cm from the LCM, which was associated with poor overall survival. The 3-year overall survival was 49% (40-57% CI) in patients with spleen greater than 15 cm below the LCM, 58% in patients with 5 cm to 14 cm, and 66% (58-74% CI) in patients with spleen less than 5 cm.
Therefore, the expert group agreed that the size of the spleen should be reduced as much as possible before transplantation. At present, the most effective drug on the market to reduce spleen size is JAKi. Ruxolitinib may be the preferred first-line option. Patients with myelofibrosis and splenomegaly more than 5 cm below the LCM or with clinical symptoms related to splenomegaly (early satiety, abdominal discomfort, pain, etc.) should receive JAKi before allo-HSCT in order to minimize the size of the spleen and improve the general state.
Although almost all patients treated with JAKi showed a certain degree of spleen reduction, about 70% of patients who responded to treatment eventually developed drug resistance [13, 14]. These high-risk patients usually have a poor prognosis. Splenectomy can be considered for such advanced patients. Splenectomy can physically reduce tumor load and improve clinical symptoms such as haemocytopenia, portal hypertension, abdominal distension and pain in about 30%-50% of patients [15, 16]. Splenectomy has the risk of increased recurrence rate and perioperative complications, but for advanced patients, when the size of spleen under the left costal margin is larger than 15 cm, splenectomy has a beneficial effect on OS, but no statistically significant adverse effect on the recurrence rate [17].
For patients with surgical contraindications (such as severe thrombocytopenia or high surgical risk), splenic irradiation or selective splenic artery catheterization can be considered for partial splenic embolization (PSE).
In summary, EBMT recommends that for patients with disease-related symptoms of splenomegaly≥ 5 cm or < 5 cm below the LCM, the maximum tolerable dose of oral JAKi should be 3 months or more. If the spleen shrinks to the left costal margin 5 cm, and the symptoms are improved, allo-HSCT can be considered. If there is no response to treatment, or if the spleen is enlarged to 4-15 cm below the left costal margin, allo-HSCT or replacement of JAKi, splenectomy, splenic irradiation or entering clinical trials can be considered. If there is no therapeutic response or the disease continues to progress to the spleen above the LCM 15 cm, the replacement of JAKi, splenectomy, splenic irradiation or entering clinical trials should be considered first, followed by allo-HSCT.
The two patients we reported all lost treatment response after the initial effective treatment with ruxolitinib, the spleen was progressively enlarged to more than 20 cm, and the spleen was resected before allo-HSCT. No serious complications occurred after the operation, hematopoietic stem cells were successfully implanted, and the primary disease was alleviated continuously after transplantation. Consistent with EBMT recommendations.
Article Info
Article Type
Case ReportPublication history
Received: Mon 13, May 2024Accepted: Tue 11, Jun 2024
Published: Tue 30, Jul 2024
Copyright
© 2023 Lizhen Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2024.02.01
Author Info
Zhengxiang Yang Aibin Zhang Xiaoyu Lai Luxin Yang Yishan Ye Qingna Guo Yibo Wu He Huang Yi Luo Lizhen Liu
Corresponding Author
Lizhen LiuBone Marrow Transplantation Center, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Figures & Tables



References
1. Cervantes F,
Dupriez B, Pereira A, Passamonti F, Reilly JT et al. (2009) New prognostic
scoring system for primary myelofibrosis based on a study of the International
Working Group for Myelofibrosis Research and Treatment. Blood 113:
2895-2901. [Crossref]
2. Mesa RA, Li CY,
Schroeder G, Tefferi A (2001) Clinical correlates of splenic histopathology and
splenic karyotype in myelofibrosis with myeloid metaplasia. Blood 97:
3665-3667. [Crossref]
3. Shimomura Y, Hara
M, Katoh D, Hashimoto H, Ishikawa T (2018) Enlarged spleen is associated with
low neutrophil and platelet engraftment
rates and poor survival after allogeneic stem cell transplantation in patients with
acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol 97:
1049-1056. [Crossref]
4. Cervantes F,
Dupriez B, Pereira A, Passamonti F, Reilly JT et al. (2009) New prognostic
scoring system for primary myelofibrosis based on a study of the International
Working Group for Myelofibrosis Research and Treatment. Blood 113:
2895-2901. [Crossref]
5. Wanless IR,
Peterson P, Das A, Boitnott JK, Moore GW et al. (1990) Hepatic vascular disease
and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia:
a clinicopathological study of 145 patients examined at autopsy. Hepatology
12: 1166-1174. [Crossref]
6. Polverelli N, Mauff
K, Kröger N, Robin M, Beelen D et al. (2021) Impact of spleen size and
splenectomy on outcomes of allogeneic hematopoietic cell transplantation for
myelofibrosis:A retrospective analysis by the chronic malignancies working
party on behalf of European society for blood and marrow transplantation
(EBMT). Am J Hematol 96: 69-79. [Crossref]
7. Rialon KL, Speicher
PJ, Ceppa EP, Rendell VR, Vaslef SN et al. (2015) Outcomes following
splenectomy in patients with myeloid neoplasms. J Surg Oncol 111:
389-395. [Crossref]
8. Wong KM, Atenafu
EG, Kim D, Kuruvilla J, Lipton JH et al. (2012)
Incidence and risk factors for early hepatotoxicity and its impact on survival
in patients with myelofibrosis undergoing allogeneic hematopoietic cell
transplantation. Biol Blood Marrow Transplant 18: 1589-1599. [Crossref]
9. Zimran E, Tripodi
J, Rampal R, Rappoport F, Zirkiev S et al. (2018) Genomic characterization of
spleens in patients with myelofibrosis. Haematologica 103: e446-e449. [Crossref]
10. Prakash S, Hoffman
R, Barouk S, Wang YL, Knowles DM et al. (2012) Splenic extramedullary
hematopoietic proliferation in Philadelphia chromosomenegative
myeloproliferative neoplasms: heterogeneous morphology and cytological
composition. Mod Pathol 25: 815-827. [Crossref]
11. Barosi G,
Ambrosetti A, Centra A, Falcone A, Finelli C et al. (1998) Splenectomy and risk
of blast transformation in myelofibrosis with myeloid metaplasia. Italian
Cooperative Study Group on Myeloid with Myeloid Metaplasia. Blood 91:
3630-3636. [Crossref]
12. Polverelli N,
Hernández Boluda JC, Czerw T, Barbui T, D'Adda M et al. (2023) Splenomegaly in
patients with primary or secondary myelofibrosis who are candidates for
allogeneic hematopoietic cell transplantation: a Position Paper on behalf of
the Chronic Malignancies Working Party of the EBMT. Lancet Haematol 10:
e59-e70. [Crossref]
13. Harrison CN, Schaap
N, Mesa RA (2020) Management of myelofibrosis after ruxolitinib failure. Ann
Hematol 99: 1177-1191. [Crossref]
14. Verstovsek S, Mesa
RA, Gotlib J, Gupta V, DiPersio JF et al. (2017) Long-term treatment with
ruxolitinib for patients with myelofibrosis: 5-year update from the randomized,
double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol
10: 55. [Crossref]
15. Zimran E, Tripodi
J, Rampal R, Rappoport F, Zirkiev S et al. (2018) Genomic characterization of
spleens in patients with myelofibrosis. Haematologica 103: e446-e449. [Crossref]
16. Mesa RA, Nagorney DS, Schwager S, Allred J, Tefferi A et al. (2006) Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer 107: 361-370. [Crossref]
17. Polverelli N, Mauff K, Kröger N, Robin M, Beelen D et al. (2021) Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: a retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT). Am J Hematol 96: 69-79. [Crossref]
