Surgical Management of Pelvic Organ Prolapse in a Woman with Right Pelvic Kidney
A B S T R A C T
A thorough knowledge of both normal and variant abdominopelvic anatomy is important to the surgeon treating patients with pelvic organ prolapse and pathology in the pelvis. Knowledge of the location of the kidney and ureters is mandatory in treating women with pelvic organ prolapse and a pelvic kidney. This case report describes surgical management for a woman with the right pelvic kidney and symptomatic pelvic organ prolapse.
Keywords
Pelvic organ prolapse, pelvic kidney
Introduction/Background
A pelvic kidney results if ascending of the kidney in the early development stages fails. This developmental failure leaves the kidney to remain in the pelvis, instead of the abdomen. In most cases, a pelvic kidney is an incidental finding and is usually asymptomatic [1]. The incidence of pelvic kidney has been approximated at 1 in 1000 births [2, 3]. Because of the greater risk of injuring aberrant vessels or overlying abdominal viscera and nerves, the pelvic kidney presents special treatment challenges [4].
Although there are some studies published on pelvic reconstruction surgery in renal transplant recipients, there is no article available on how to manage a woman with pelvic organ prolapse (POP) with a pelvic kidney [5-7]. Despite the rarity of pelvic kidney, it is imperative to be aware of this anatomical variation when managing a patient with pelvic kidney. This case report describes the management of POP in a woman with a right pelvic kidney.
Case Presentation
A 51-year-old female gravida 3 and parity 3 in the perimenopausal status presented for the symptomatic stage 3 POP. Past medical histories included well-controlled asthma, gastric acid reflux, and hyperlipidemia. No significant past medical or surgical histories except for the known congenital right pelvic kidney. Intake vital signs were all within normal limits. Physical exam revealed fullness without tenderness in the right pelvic area. Pelvic Organ Prolapse Quantification (POPQ) was noted to be +1, +2, +2/6, 3, 9/-1, -1, -1. Post void residual urinary volume was 20cc. Urine analysis was within normal limits without evidence of infection or inflammation.
Case Management
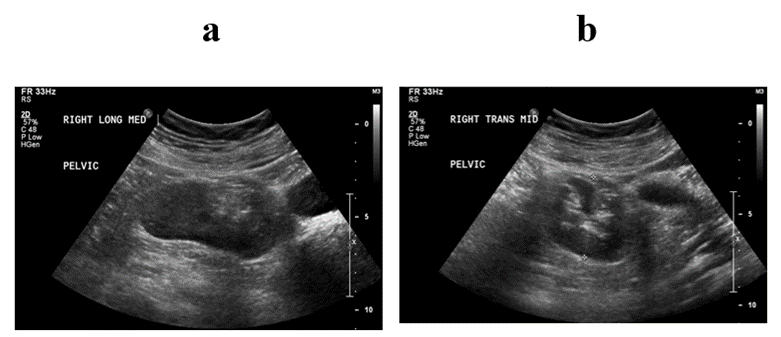
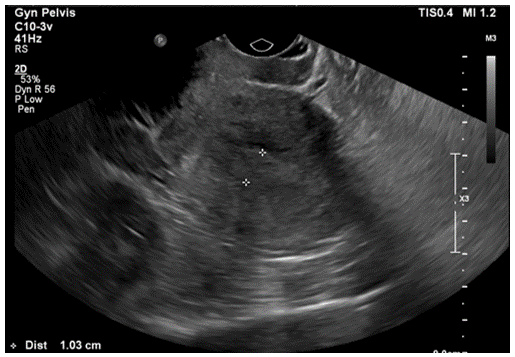
After routine counseling for POP management with risks, benefits and alternatives (R/B/A), the patient declined the conservative management options (i.e., observation, pelvic floor therapy, or pessary trial) but desired to proceed for surgical management. Patient voiced understanding on mesh complications and expressed her desire for sacrocolpopexy (SCP) with removal of the uterus, stating that she would like the most durable procedure to address her prolapse. Patient was counseled that it might not be possible to perform SCP if the right pelvic kidney is in the pelvic area where the SCP mesh arm would be positioned. Patient was informed that if the intraoperative findings were not optimal for the SCP procedure, the apical suspension should be performed with other options, like either uterosacral ligament suspension (USLS) or sacrospinous ligament fixation (SSLF). R/B/A for each apical suspension was also discussed in detail. Preoperative urodynamic testing with POP reduction did not reveal occult stress urinary incontinence (SUI) or detrusor overactivity. The bladder capacity was noted to be 255cc. As a part of the preoperative work up, renal and pelvic ultrasounds were performed. Renal ultrasound was consistent with the right pelvic kidney without stones or hydronephrosis (see Figures 1a & 1b). Transvaginal ultrasound revealed an enlarged uterus (10.3cm × 5.8cm × 4.0cm) with an ill-defined endometrial lining of 10mm thickness (see Figure 2). Both ovaries appeared within normal limits. Considering perimenopausal status and thickened endometrial lining, office endometrial biopsy was performed and resulted in “complex hyperplasia with atypia, cannot rule out adenocarcinoma”. To further evaluate the endometrial abnormality, hysteroscopic dilation and curettage was performed under anaesthesia after R/B/A discussion: The final pathology showed focal complex hyperplasia with atypia. Gynaecologic Oncology was consulted to manage this finding. The recommendations were i) to proceed to planning of POP procedure, ii) to send a frozen section for the uterus intraoperatively, iii) if the frozen section is normal, there is no need to remove ovaries.
Figure 1: Right pelvic kidney on Renal Ultrasound. a) Longitudinal View, b) Transverse View.
Figure 2: Thickened Endometrium on Transvaginal Ultrasound.
The patient consented and signed the form with a witness present for exam under anaesthesia; robotic-assisted total laparoscopic hysterectomy, bilateral salpingectomy, possible bilateral oophorectomy (if the intraoperative frozen section consistent with malignancy), SCP vs. USLS vs. SSLF with vaginal repairs, cystoscopy, simple cystometrogram after R/B/A discussion.
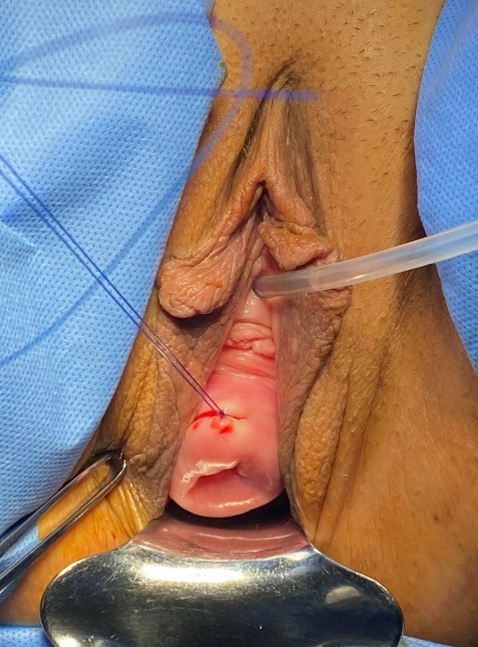
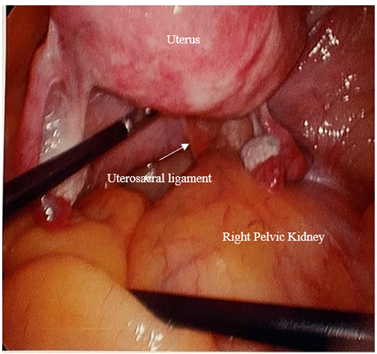
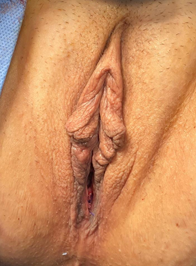
Exam under anaesthesia showed normal external female genitalia with age-appropriate atrophy and Stage 3 POP with atrophic vagina (see Figure 3). The uterus was noted to be 12 cm in size and boggy, but mobile. Right side pelvic fullness was noted. The scout cystoscopy was performed: The right ureteral orifice was visualized in the symmetric position from the left orifice. Brisk ureteral flow was confirmed bilaterally. After removal of cystoscopy, the bladder was drained then a Foley urinary catheter was placed in a sterile fashion. Laparoscopic survey revealed a 12cm boggy uterus with no distinctive fibroids/mass and normal appearing fallopian tubes except for surgically absent ampullary area bilaterally and normal appearing ovaries bilaterally. The right pelvic kidney occupied the entire right pelvic area, blocking safe access to the sacral promontory or proximal portion of uterosacral ligaments (see Figure 4). Frozen section from the removed uterus/cervix returned as benign adenomyosis.
Figure 3: The preoperative presentation with stage 3 pelvic organ prolapse.
Figure 4: Intraoperative findings.
An intraoperative decision was made to proceed to vaginal repair with sacrospinous ligament suspension with vaginal wall repair, after laparoscopic hysterectomy with bilateral salpingectomy was completed. The robot was undocked after reassuring excellent hemostasis from all hysterectomy surgical sites. The vaginal procedure was as follows: the vaginal cuff was identified then whipped stitches were performed using 0-absorbable suture to ensure hemostasis from the vaginal cuff. The anterior vaginal mucosa was then palpated to locate the bladder neck. The anterior vaginal wall under the bladder neck was grasped with Allis clamps. The anterior vaginal cuff was also grasped with Allis clamps. After injection of 1% lidocaine with epinephrine submucosally, the vaginal epithelium was carefully dissected off from the underlying endopelvic fascia using sharp and blunt dissection. Using 2-0 delayed absorbable suture, several U stitches were placed at the vesicovaginal junction to reduce the cystocele. The vaginal epithelial layer was reapproximated using 2-0 absorbable suture. After draining the bladder via a straight catheter, the cystoscopy was repeated, revealing the normal findings again. The posterior vaginal epithelium was grasped bilaterally at the hymen level with Allis clamps and 1% lidocaine with epinephrine was injected for hydrodissection and hemostasis.
A transverse incision between the two Allis clamps was made, followed by a longitudinal incision from the level of the posterior fourchette to the posterior vaginal cuff: then, the posterior vaginal epithelium was dissected off of the underlying rectovaginal fascia using sharp and blunt dissection using dissecting scissors to the level of the sulci laterally and to the level of the posterior vaginal cuff. The right side pararectal space was entered with sharp and blunt dissection to the level of the ischial spine (IS). The right sacrospinous ligament was identified on the right IS: the ligament was traced down to the spine level. The overlying tissues were bluntly dissected away. Using the Capio device with a 0 permanent suture, the sacrospinous ligament was grasped at 2 cm medial to the IS, while avoiding adjacent structures. The same steps were repeated to grasp the ligament 1cm medial to the first suspension suture. The ends of the sutures were then tagged at this time. The enterocele was reduced using 2-0 delayed absorbable sutures in a purse-string fashion. Rectocele was reduced with several interrupted stitches of 2-0 delayed absorbable suture in U stitch-fashion. Excess posterior vaginal epithelium was trimmed and sent to pathology.
The previously tagged 0 permanent sacrospinous suspension sutures were attached submucosally on the posterior vaginal cuff, using a free needle. The vaginal cuff was reapproximated using 0 absorbable suture in several Figure of 8 stitches. The posterior vaginal epithelium was reapproximated with 2-0 absorbable sutures in a running fashion to the level of the pararectal dissection level then the needle was protected for the later use. The posterior vaginal cuff was suspended using previously tagged 0 permanent sacrospinous suspension sutures. No suture bridges were noted between the tissues. The bulbocavernosus muscles and deep transverse perineal muscles were separately plicated in the midline to reconstruct the perineal body, using 0 absorbable suture. The remainder of the vaginal epithelium and perineum skin was reapproximated with 2-0 absorbable suture in a running fashion. The final cystoscopic evaluation revealed bubbles on the dome, normal bladder mucosa but mild trabeculation globally, brisk ureteral flow bilaterally, normal urethra, free of stitches or mass. The right ureteral orifice was visualized in the symmetric position from the left orifice. Total vaginal length at the end of the procedure was noted as 8~9cm. Digital rectal exam at the end of the procedure was within normal limits and free of stitches. The vaginal cuff was well suspended to the right SSL without a suture bridge. Excellent hemostasis from all surgical pedicles and vaginal walls at the end of the procedure. The bladder was drained then a fresh foley catheter was placed in a sterile fashion. All surgeons changed their gowns and gloves.
The patient was replaced in the Trendelenburg position then the pneumoperitoneum was recreated. Using 0-degree regular laparoscopic scope, another survey was performed, confirming excellent hemostasis from all the surgical pedicles. Patient was placed back to the supine position. All trocars and instruments were removed from the abdomen after evacuating CO2 gas from the abdomen. The skin layer was reapproximated with 4-0 absorbable suture, followed by Steris Strips, then 2×2 gauze, and Opsite dressing were applied. Excellent hemostasis was noted. The patient tolerated the procedure well. Sponge, lap, and needle counts were correct ×2. The patient was transferred to recovery in stable and awake condition. The final presentation of her POP repair is shown in (Figure 5). Patient passed an active void trial via simple cystometrogram before her discharge to home on the same day.
Figure 5: The final presentation after the POP repair.
At 2 weeks postoperative visit, the patient was recovering well, without concerns. At 3 months postoperative visit, the patient was very happy and satisfied with the surgical outcomes. The postvoid residual was noted to be 10cc. The vagina appeared to be well supported with the postoperative POPQ: -2, -2, -8/3, 3, 9/-3, -3, x.
Discussion
A thorough knowledge of both normal and variant abdominopelvic anatomy is important to the surgeon treating patients with POP and pathology in the pelvis. Knowledge of the location of the kidney and ureter is mandatory in treating women with POP and pelvic kidney, especially in cases of extensive pelvic dissection, retractor placement, mesh placement or passage of a retropubic trocar, etc.: therefore, preoperative evaluation is recommended with imaging and/or cystoscopy checking on ureteral patency [5]. For the presenting case, preoperative renal and transvaginal ultrasounds were performed to confirm the gross location of the right pelvic kidney and to rule out any other anomalies in urinary/reproductive organs. In addition, the surveillance cystoscopy was performed at the beginning of the procedure, revealing normal ureteral orifice location and ureteral patency.
Although pelvic kidneys are usually asymptomatic and are found incidentally, they can be associated with other urinary conditions such as nephrolithiasis, ureteropelvic junction obstruction, and extrarenal calyces [1]. Some consider arteriography very beneficial in identifying the variant arterial supply to prevent blood loss and nephrotomography helpful in viewing the parenchyma and the collecting system [8].
It is also known that the pelvic kidney can be associated with other congenital anomalies like vaginal atresia, imperforate hymen, hypoplastic uteri, and rudimentary fallopian tube and ovary [8]. This happens because urinary and reproductive organs share the same embryologic origin (the mesonephric duct). Thus, it is recommended to have reproductive organs get evaluated by thorough physical examination and imaging if urinary congenital anomalies are present. Fortunately, no other structural congenital anomalies were noted in our presenting case, except for the right pelvic kidney.
Up to date, there are no published data available regarding the safety/efficacy/choice of POP repair surgical procedures in women with a congenital pelvic kidney, although there are some published articles available in renal transplant patients [5-7]. It appears that POP surgery in renal transplanted women is technically possible with reportedly good outcomes. Shveiky et al. (2009) presented a case series of 5 pelvic reconstruction cases in kidney transplant recipient women; three women received transvaginal hysterectomy with vaginal repair with or without mid-urethral sling, while one woman had only anterior repair and the other woman had only mid-urethral sling [5]. Three out of 5 women received a mid-urethral sling (two with transobturator sling and one with retropubic sling). For 3 to 36 months follow-up period, no complications were reported. Hoda et al. (2010) presented 16 female renal transplant recipients with POP with or without SUI: a total of 12 anterior and 4 combined anterior/posterior colporrhaphies were performed [6]. A concomitant transobturator sling procedure was performed on 8 women. None of the patients received hysterectomy or any apical suspension at the time of the procedure. At 12 months follow-up, only 25% (4 out of 16 women) exhibited stage 1 POP. Unlike the case series by Shveiky et al. (2009) and Hoda et al. (2010), which present vaginal repairs only in renal transplant patients, Rouffilange et al. (2017) reports a laparoscopic double mesh SCP in a 73-year-old woman with a grafted kidney [7]. Despite immunosuppression due to anti-injection treatments, the postoperative course was uneventful. SCP for this particular patient was likely feasible because the transplanted kidney was in the left iliac fossa.
Since there is scarce data available on how to manage a woman with POP with a pelvic kidney, the patient in this presenting case report was managed based on the limited data on women with POP and a transplanted kidney. Several points that were discussed with the patient preoperatively include the followings: i) The intraoperative surgical risks associated with POP repair in women with a pelvic kidney are likely similar to women with a transplanted kidney because of their location in the pelvis; ii) Although the vaginal approach with natural tissue repair has been a mainstream of POP surgical repair for women with a transplanted kidney, laparoscopic SCP with mesh may be an option as well despite the immunosuppression status; iii) Compared to women with a transplanted kidney and in the presence of immunosuppressive treatments, women with a congenital pelvic kidney would less likely suffer from impaired wound healing or prosthesis infection from the laparoscopic SCP; iv) Compared to the vaginal approach, the laparoscopic approach would provide more precise surveillance of the pelvic structures and better aid hysterectomy and adnexa removal (for the presence of complex hyperplasia with atypia), under direct visualization, while avoiding damage to the pelvic kidney, and v) Even if the laparoscopic apical suspension after hysterectomy was planned, it might not be feasible depending on the intraoperative findings and vaginal apical suspension with vaginal wall repairs would be necessary [5-7]. Thorough preoperative counseling and surgical planning are necessary to manage women with a congenital pelvic kidney and symptomatic POP.
Conclusion
Despite the rarity of pelvic kidney, it is imperative to be aware of this anatomical variation when managing the patient with POP and a pelvic kidney. Thorough preoperative counseling is the key to the patient’s satisfaction.
Consent
The authors certify they have obtained the verbal and written patient consent. The patient has given her verbal and written consent for her images and other clinical information to be reported in the journal. The patient understands her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 01, Aug 2022Accepted: Fri 26, Aug 2022
Published: Fri 09, Sep 2022
Copyright
© 2023 Woojin Chong. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2022.03.01
Author Info
Corresponding Author
Woojin ChongDepartment of Obstetrics and Gynecology, Inspira Health Network, Vineland, New Jersey, USA
Figures & Tables
References
1. Eid S, Iwanaga J,
Loukas M, Oskouian RJ, Tubbs RS (2018) Pelvic Kidney: A Review of the
Literature. Cureus 10: e2775. [Crossref]
2. Bingham G, Leslie
SW (2022) Pelvic Kidney. In: StatPearls. Treasure Island (FL): StatPearls
Publishing.
3. National Institute
of Diabetes and Digestive and Kidney Diseases (2019) Ectopic Kidney.
4. Goel A, Dalela D
(2007) Re: Cinman NM, Okeke Z, Smith AD. Pelvic kidney: Associated diseases and
treatment. J Endourol 2007;21:836-842. J Endourol 22: 157-158. [Crossref]
5. Shveiky D, Blatt A,
Sokol AI, Nghiem HG, Iglesia CB (2009) Pelvic reconstructive surgery in renal
transplant recipients. Int Urogynecol J Pelvic Floor Dysfunct 20:
551-555. [Crossref]
6. Hoda MR, Wagner S,
Greco F, Heynemann H, Fornara P (2010) Pelvic organ prolapse management in
female kidney transplant recipients. J Urol 184: 1064-1068. [Crossref]
7. Rouffilange J, Deslandes M, Lopez L (2017) Laparoscopic Management of Pelvic Organ Prolapse in a Kidney Transplant Recipient. Urol Case Rep 13: 145-146. [Crossref]
8. Dretler SP, Olsson C, Pfister RC (1971) The anatomic, radiologic and clinical characteristics of the pelvic kidney: an analysis of 86 cases. J Urol 105: 623-627. [Crossref]
