Survival Outcomes in Stage IV Bladder Cancer Patients Treated with Cisplatin/Gemcitabine Versus Carboplatin/Gemcitabine: A Retrospective Analysis in Veteran Patients
A B S T R A C T
Purpose: Gemcitabine/cisplatin (GCi) is the standard regimen used to treat stage IV urothelial bladder cancers. However, most of the bladder cancer patients are older, with poor performance status and renal dysfunction, and are not eligible for cisplatin-containing regimens. There are no randomized studies comparing gemcitabine/carboplatin (GC) and gemcitabine/cisplatin (GCi).
Methods: We identified stage IV bladder cancer patients treated within the Veterans Health Administration (VHA), healthcare system between January 2000 and December 2010 from Veterans Affairs Central Cancer Registry (VACCR). Overall survival (OS) was visualized using Kaplan-Meier curves and tested for the significance of the treatment-arm difference using the log-rank test.
Results: There were 196 patients with stage IV bladder cancer, out of which 78 patients were treated with GC and 118 patients treated with GCi. The median OS for all patients was 12.5 months a 95% confidence interval (CI) of 10.0-14.6 months. The median OS for patients treated with GC was 13.4 months (95% CI 9.8-17.5 months), and that of the patients treated with GCi was 11.7 months (95% CI 9.3-14.9 months). Cox regression revealed equal group mortality rates, with GC having a (hazard ratio (HR) of 0.96 (CI 0.72-1.27; P= 0.81)) compared to GCi.
Conclusion: Our study is the largest comparing GC and GCi in stage IV urothelial bladder cancer patients. It showed that there is no difference in OS in patients treated with GC and GCi.
Introduction
Bladder cancer is the second most common cancer of the genitourinary tract, with approximately 350, 000 new cases worldwide. The American Cancer Society reported 74,000 new cases and 11,000 deaths from bladder cancer as of 2015 [1]. Transitional cell carcinoma (TCC) is the most predominant histological type, and represents more than 90% of cases. 50% of the patients with invasive disease will develop metastasis. Cisplatin-based chemotherapy regimens are used in the treatment of metastatic urothelial bladder cancer [2-4]. Because of the toxicity profile, GCi is the preferred therapy compared to other regimens, and yields a median survival of 15 months [2]. However, most of the patients with metastatic bladder cancer are older, with comorbidities, including renal insufficiency (creatinine clearance of < 60ml/min) and poor performance, and therefore are not eligible for cisplatin-based chemotherapy [4, 5]. Alternately, carboplatin is often substituted for cisplatin. Studies have shown that GC has a better toxicity profile compared to other regimens, and hence, it is the standard regimen used to treat advanced bladder cancer patients, unfit for cisplatin therapy.
To date, there is no prospective phase III study comparing GC and GCi in TCC of bladder. We conducted a retrospective cohort study to compare survival outcomes in stage IV TCC of the bladder treated with GC and GCi in veteran population between January 2000 to December 2010.
Materials and Methods
This study was approved by the institutional review board of Central Arkansas Veterans Healthcare System (CAVHS), Little Rock. Patients with stage IV transitional carcinoma of the bladder, treated with either GCi or GC, between January 2000 and December 2010 nationally in all VA centers, were identified using the department of Veterans Affairs Central Cancer Registry (VACCR). International Classification of Diseases Ninth Revision (ICD-9) was used to identify bladder cancer patients. Stage IV patients were identified using the seventh edition American Joint Committee on Cancer (AJCC) and tumor, lymph node and metastasis (TNM) staging.
Statistical Analysis
The treatment arms were assessed for imbalances in patient characteristics, using Wilcoxon’s rank-sum test for age and the chi-square test for sex, race, tobacco history, histology, cancer status, and sites of first, second, and third metastases. Overall survival was visualized using Kaplan-Meier curves, tested for the significance of the treatment-arm difference using the log-rank test, and evaluated for the magnitude of the treatment-arm difference using Cox regression.
Table 1: Patient Characteristics.
|
Differences in Patient Characteristics |
|||
|
Characteristics |
No of Patients cisplatin/Gemcitabine 118 |
No of Patients Carboplatin/Gemcitabine 78 |
Total No of Patients 196 |
|
Age Mean SD |
61 7.75 |
67 10.8 |
63 9.46 |
|
Sex Male Female |
116 (98%) 2 (2%) |
78 (100%) 0 (0%) |
194 (99%) 2 (1%) |
|
Histology Transitional Squamous Adeno Nos Giant Cell |
109 (92%) 4 (3%) 3 (3%) 1 (1%) 1 (1%) |
73 (94%) 2 (3%) 0 (0%) 2 (3%) 0 (0%) |
182 (93%) 6 (3%) 3 (1.5%) 3 (1.5%) 1 (0.5%) |
|
Race White Black American Indian Unknown |
103 (87%) 12 (10%) 2 (2%) 1 (1%) |
68 (87%) 9 (12%) 0 (0%) 1 (1%) |
171 (87%) 21 (11%) 2 (1%) 2 (1%) |
|
Tobacco History Current smoker Previous smoker Never smoked Unknown |
67 (57%) 33 (28%) 7 (6%) 11 (9%) |
23 (29%) 34 (44%) 15 (19%) 6 (8%) |
90 (46%) 67 (34%) 22 (11%) 17 (9%) |
|
Sites of Metastasis Lung Bone Lymph node Liver Local metastasis peritoneum Unknown Missing |
30 (25%) 25 (21%) 27 (23%) 10 (8.5%) 7 (6%) 2 (2%) 14 (12%) 3 (2.5%) |
18 (23%) 21(27%) 18 (23%) 8 (10%) 3 (4%) 2 (3%) 8 (10%) 0 |
48 (25%) 46 (23%) 45 (23%) 18 (9%) 10 (5%) 4 (2%) 22 (11%) 3 (1.5%) |
Results
There were 196 patients with stage IV bladder cancer, out of which 102 (92%) had transitional cell carcinoma and the rest had other histologies (Table 1). 78 patients were treated with GC and 118 treated with GCi. The median age of patients who received GCi was 61 and the median age of patients who received GC was 67. 99% of the patients were male, and one percent were female. Differences in patient characteristics were outlined in (Table 1).
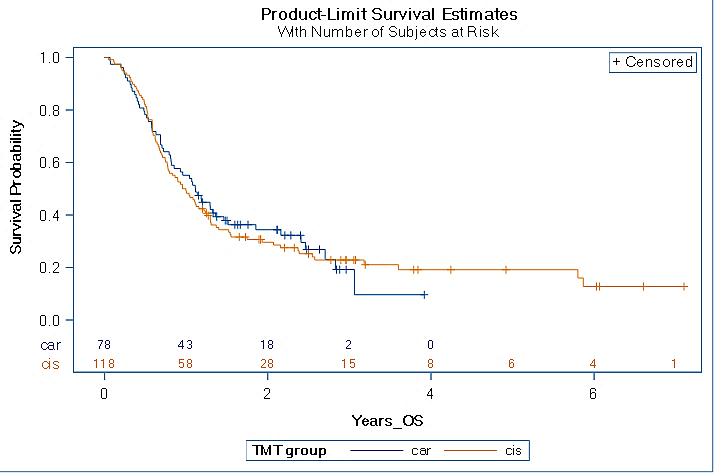
The median survival for all patients treated with GC and GCi was 12.5 months (95% confidence interval (CI) 10.0-14.6 months). The median overall survival for patients treated with GC was 13.4 months (95% CI 9.8-17.5 months), and that of patients treated with GCi was 11.7 months (95% CI 9.3-14.9 months). Cox regression revealed equal group mortality rates, with GC having a hazard ratio (HR) of 0.96 (90% CI 0.72-1.27; P=0.81) compared to GCi (Figure 1).
Figure 1: Kaplan-meier curve for overall survival. Cox regression revealed equal group mortality rates, with GC having a hazard ratio (HR) of 0.96 (90% CI 0.72-1.27; P=0.81) compared to GCi.
Discussion
GCi is the preferred first-line regimen used in the treatment of stage IV urothelial bladder cancers [6]. However, most of the metastatic bladder cancer patients are older and have several comorbidities precluding the use of cisplatin. Eastern Cooperative Oncology Group performance status of greater than 2, creatinine clearance less than 60 mL/min, grade 2 hearing loss, grade 2 neuropathy, and/or New York Heart Association Class III heart failure are the criteria for cisplatin ineligibility according to a survey based on genitourinary medical oncologists [5]. According to a study, more than 40% of the patients with advances urothelial bladder cancer over age 70 were ineligible for cisplatin-based treatment [7]. Phase II studies have shown that carboplatin-based regimens have activity in metastatic urothelial carcinoma and are tolerated well [6, 8-13]. An EORTC trial of 238 patients compared carboplatin/gemcitabine (GC) to methotrexate/carboplatin/vinblastine (M-CAVI) in advanced urothelial cancer patients unfit for cisplatin, and showed median overall survival (OS) of 9.3 months in with GC versus 8.1 months with MCAVI. MCAVI had more toxicity [9]. Hence GC is the standard for cisplatin-ineligible metastatic bladder cancer patients. Like in other cancers, immune therapy with antibodies directed against programmed cell death protein 1 (PD1) and its ligand (PDL1) has revolutionized the treatment of metastatic bladder cancers. FDA has approved PD1 and PDL1 inhibitors in patients in the front line setting in patients who are ineligible for platinum therapy and in cisplatin-ineligible patients with PD1 positivity [6].
To date, there are only two small retrospective studies and one small randomized study comparing GC and GCi. The first study is a retrospective study of 44 from Korea comparing toxicity and response between split-dose cisplatin and gemcitabine and carboplatin and gemcitabine. There was no difference in toxicity between arms, but the GCi arm showed significantly higher response rate (68.4 %) compared to GC (31.6 %) (p = 0.023) [11]. Another retrospective study of 41 patients from Japan showed median overall survival and progression-free survival in gemcitabine with split-dose cisplatin versus GC was 18.1 versus 12.5 months (p=0.0454) and 9.9 versus 6.4 months (p=0.0404), respectively [12]. A small phase II randomized study of 110 patients comparing GC and GCi in locally advanced and metastatic TCC from Italy showed similar toxicity profiles along with median survivals of 12.8 months and 9.8 months in GCi and GC, respectively [13]. This study used three weekly regimens as opposed to a four-week regimen, and was not powered to assess overall survival [13].
However, there are no randomized studies in North America comparing GC and GCi. Our study is a larger study and included all VHA patients with stage IV bladder cancers treated with GC and GCi. Our study showed that there was no difference in overall survival in stage IV urothelial bladder cancer patients treated with GC versus GCi. The median OS for patients treated with GC was 13.4 months (95% CI 9.8-17.5 months) and that of patients treated with GCi was 11.7 months (95% CI 9.3-14.9 months). Cox regression revealed equal group mortality rates, with GC having a hazard ratio (HR) of 0.96 (90% CI 0.72-1.27; P=0.81) compared to GCi.
There are limitations to our study. Ours is a retrospective analysis of data obtained from the Veterans Affairs Central Cancer Registry. Fewer patients were treated with GCi and GC compared to other regimens. We could not get information about the number of cycles of chemotherapy and the dose of chemotherapy that the patients received from the database.
In conclusion, our study is the largest study comparing GC and GCi in stage IV urothelial bladder cancer patients. It showed that there is no difference in OS in patients treated with GC and GCi. Our study is hypothesis-generating, and further randomized studies comparing GC and GCi are needed to answer if carboplatin can be substituted for cisplatin. However, it would be hard to enroll cisplatin eligible patients to the GC arm. A randomized study of split-dose cisplatin and gemcitabine and carboplatin and gemcitabine with or without PDL1 inhibitors in patients ineligible for full-dose cisplatin would a more feasible study to enroll patients.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 13, May 2020Accepted: Fri 29, May 2020
Published: Fri 05, Jun 2020
Copyright
© 2023 Anuradha Kunthur. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.06.02
Author Info
Anuradha Kunthur Eric Siegel Rangaswamy Govindarajan
Corresponding Author
Anuradha KunthurAssistant Professor, Division of Hematology Oncology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
Figures & Tables
Table 1: Patient Characteristics.
|
Differences in Patient Characteristics |
|||
|
Characteristics |
No of Patients cisplatin/Gemcitabine 118 |
No of Patients Carboplatin/Gemcitabine 78 |
Total No of Patients 196 |
|
Age Mean SD |
61 7.75 |
67 10.8 |
63 9.46 |
|
Sex Male Female |
116 (98%) 2 (2%) |
78 (100%) 0 (0%) |
194 (99%) 2 (1%) |
|
Histology Transitional Squamous Adeno Nos Giant Cell |
109 (92%) 4 (3%) 3 (3%) 1 (1%) 1 (1%) |
73 (94%) 2 (3%) 0 (0%) 2 (3%) 0 (0%) |
182 (93%) 6 (3%) 3 (1.5%) 3 (1.5%) 1 (0.5%) |
|
Race White Black American Indian Unknown |
103 (87%) 12 (10%) 2 (2%) 1 (1%) |
68 (87%) 9 (12%) 0 (0%) 1 (1%) |
171 (87%) 21 (11%) 2 (1%) 2 (1%) |
|
Tobacco History Current smoker Previous smoker Never smoked Unknown |
67 (57%) 33 (28%) 7 (6%) 11 (9%) |
23 (29%) 34 (44%) 15 (19%) 6 (8%) |
90 (46%) 67 (34%) 22 (11%) 17 (9%) |
|
Sites of Metastasis Lung Bone Lymph node Liver Local metastasis peritoneum Unknown Missing |
30 (25%) 25 (21%) 27 (23%) 10 (8.5%) 7 (6%) 2 (2%) 14 (12%) 3 (2.5%) |
18 (23%) 21(27%) 18 (23%) 8 (10%) 3 (4%) 2 (3%) 8 (10%) 0 |
48 (25%) 46 (23%) 45 (23%) 18 (9%) 10 (5%) 4 (2%) 22 (11%) 3 (1.5%) |
References
- Seigel R, Miller K, Jemal A (2015) Cancer Statistics 2015. CA Cancer J Clin 65: 5-29. [Crossref]
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L et al. (2005) Long-term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol 23: 4602-4608.[Crossref]
- Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD et al. (2006) Seven Year Update of an EORTC Phase III Trial of High-Dose Intensity M-VAC Chemotherapy and G-CSF Versus Classic M-VAC in Advanced Urothelial Tract Tumours. Eur J Cancer 42: 50-54. [Crossref]
- Loehrer PJ Sr, Einhorn LH, Elson PJ, Crawford ED, Kuebler P et al. (1992) A Randomized Comparison of Cisplatin Alone or in Combination With Methotrexate, Vinblastine, and Doxorubicin in Patients With Metastatic Urothelial Carcinoma: A Cooperative Group Study. J Clin Oncol 10: 1066-1073. [Crossref]
- Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T et al. (2011) Treatment of Patients With Metastatic Urothelial Cancer "Unfit" for Cisplatin-based Chemotherapy. J Clin Oncol 29: 2432-2438. [Crossref]
- Merseburger AS, Apolo AB, Chowdhury S, Hahn NM, Galsky MD et al. (2019) SIU-ICUD Recommendations on Bladder Cancer: Systemic Therapy for Metastatic Bladder Cancer. World J Urol 37: 95-105. [Crossref]
- Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM et al. (2006) Impact of Renal Impairment on Eligibility for Adjuvant Cisplatin-Based Chemotherapy in Patients With Urothelial Carcinoma of the Bladder. Cancer 1: 506-513. [Crossref]
- Bellmunt J, Ribas A, Eres N, Albanell J, Almanza C et al. (1997) Carboplatin-based Versus Cisplatin-Based Chemotherapy in the Treatment of Surgically Incurable Advanced Bladder Carcinoma. Cancer 80: 1966-1972. [Crossref]
- De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M el al. (2012) Randomized Phase II/III Trial Assessing Gemcitabine/Carboplatin and Methotrexate/Carboplatin/Vinblastine in Patients With Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. J Clin Oncol 30: 191-199. [Crossref]
- Petrioli R, Frediani B, Manganelli A, Barbanti G, De Capua B et al. (1996) Comparison Between a Cisplatin-Containing Regimen and a Carboplatin-Containing Regimen for Recurrent or Metastatic Bladder Cancer Patients. A Randomized Phase II Study. Cancer 77 : 344-351. [Crossref]
- Kim YR, Lee JL, You D, Jeong IG, Song C et al. (2015) Gemcitabine Plus Split-Dose Cisplatin Could Be a Promising Alternative to Gemcitabine Plus Carboplatin for Cisplatin-Unfit Patients With Advanced Urothelial Carcinoma. Cancer Chemother Pharmacol 76: 141-153. [Crossref]
- Izumi K, Iwamoto H, Yaegashi H, Shigehara K, Nohara T et al. (2019) Gemcitabine Plus Cisplatin Split Versus Gemcitabine Plus Carboplatin for Advanced Urothelial Cancer With Cisplatin-unfit Renal Function. In vivo 33: 167-172. [Crossref]
- Dogliotti L, Cartenì G, Siena S, Bertetto O, Martoni A et al. (2007) Gemcitabine Plus Cisplatin Versus Gemcitabine Plus Carboplatin as First-Line Chemotherapy in Advanced Transitional Cell Carcinoma of the Urothelium: Results of a Randomized Phase 2 Trial. Eur Urol 52: 134-141. [Crossref]
