Synthesis and Antimicrobial Activities of Containing Sulfur Heterocyclic Curcumin Derivatives
A B S T R A C T
Eight novel containing sulfur heterocyclic curcumins were synthesized and characterized by 1H-NMR, FT-IR and MS spectroscopy. Their antimicrobial activities against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger were also tested for MIC by using serial tube dilution method. The results showed that the antimicrobial activities of synthesized curcumin derivatives were better than curcumin. Especially, the compound 4-(1,3-dithiolan-2-ylidene)-1,7-di(thiophen-2-yl) hepta-1,6-diene-3,5-dione (2g) exhibited excellent the antimicrobial activities among these curcumin derivatives.
Keywords
Curcumins, sulfur heterocycle, synthesis, antimicrobial activity
Introduction
Analogues of curcumin are an interesting class of pharmacological organic compounds, due to their diverse biological pharmacology activities, such as anti-carcinogen, anti-oxidant, anti-inflammatory, anti-tumor, immunomodulation, chemoprevention, Alzheimer's disease, anti-microbial [1-10]. But the natural curcuminoids have high extraction cost, low extraction rate, unstable structure and low bioavailability. To improve the bioavailability of curcumin, the curcumin was modified with various substituents [11-15]. In addition, the stereoelectronic effects of heterocycles can modulate molecular conformation and impart strikingly different biological properties [14, 16]. Keeping this in view, we designed and synthesized some novel containing sulfur heterocyclic curcumin derivatives and presented their initial results of antimicrobial against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger.
Results and Discussion
I Chemistry and Spectroscopy
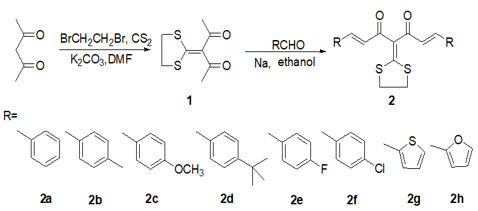
The synthesis of curcumin derivatives is conducted as outlined in (Scheme 1). The 3-(1,3-dithiolan-2-ylidene) pentane-2,4-dione (1) were prepared by the acetylacetone with carbon disulfide in N, N-dimethylformamide using potassium carbonate as the catalyst, followed by reaction with the 1,2-dibromoethane. The structures of compound (1) were established by 1H-NMR, FT-IR and MS spectroscopy. Their 1H NMR spectra exhibited the single peaks at δ= 1.75ppm attributed to the keto-CH3 protons, the triple peaks at δ= 3.52-3.75ppm corresponding to the methylene protons in sulfur heterocycle. The IR spectra data also demonstrated the presence of the C=C stretching vibrations at 1628 cm-1 in the 3-(1,3-dithiolan-2-ylidene) pentane-2,4-dione, and the presence of the C-S stretching vibrations at 1287-1277 cm-1 and 992-973 cm-1.
The curcumin derivatives (2a-2h) were synthesized via Claisen condensation of compound (1) with the required aldehyde in the presence of sodium ethoxide. Their structures were slao confirmed by FTIR, 1H NMR and mass spectroscopy. Their 1H NMR spectra revealed the double-double peaks at δ = 7.45-7.18ppm and δ = 6.97-6.81ppm, the single peaks at δ = 3.42-3.31ppm corresponding to the methylene protons in sulfur ring. Their IR spectra showed the absorption bands at 1571-1510cm-1 attributed to the C=C stretching vibration.
Scheme 1: The synthetic routes for curcumin derivatives (2a-2h).
II Anti-Bacterial Activity
The screening results of antimicrobial activity of curcumin derivatives (2a-2h) are summarized in (Table 1). It is obvious from the data that these derivatives possess inhibitory activities to a certain degree against the tested microorganisms and display better antimicrobial activities than curcumin against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger. It was also observed that the curcumin derivatives (2e-2h) exhibited much significant antimicrobial activities among these synthesized compounds, which was probably resulted from the introduction of modified substituents led to the increase of the hydrophobicity and the delocalization of the electron cloud. Especially, the derivative 2g displayed the highest activities in these synthesized compounds.
Table 1: The MIC values(µg/mL)of curcumin derivatives (2a–2h).
|
Compounds |
S. aureus |
B. subtilis |
E. coli |
A. niger |
|
2a |
64 |
64 |
64 |
32 |
|
2b |
64 |
64 |
32 |
64 |
|
2c |
32 |
64 |
64 |
32 |
|
2d |
64 |
64 |
64 |
64 |
|
2e |
16 |
8 |
16 |
8 |
|
2f |
8 |
8 |
16 |
16 |
|
2g |
8 |
8 |
8 |
8 |
|
2h |
16 |
8 |
8 |
16 |
|
Curcumin |
128 |
128 |
>256 |
>256 |
Conclusion
In conclusion, we designed and synthesized eight new curcumin derivatives. Their structures were confirmed by FT-IR, 1H NMR and MS spectroscopy. The antimicrobial activities of these curcumin derivatives were evaluated by serial tube dilution method against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger. The results showed that these derivatives showed certain degree of antimicrobial activities and their activities were much better than curcumin. In addition, curcumin derivatives (2e-2h) had much higher antimicrobial activities in these derivatives. Especially, the derivative 2g exhibited the highest antimicrobial activities against the tested microorganisms and all the MIC values were 8 µg/mL against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger. Thus, these studies provide a lead for synthesis and evaluation of more curcumin derivatives for antimicrobial activity as the same could lead to the discovery of some potential agents.
Experimental
Melting points were determined using X-4 digital melting point apparatus and are corrected by benzoic acid. Infrared spectra were recorded on a Nicolet FTIR 5700 spectrophotometer with KBr pellets. The 1H NMR spectra were measured on an advance IIITM 300 MHz NB Digital NMR spectrometer. Electrospray ionization mass spectra (ESI–MS) were performed with a Finnigan LCQ Advantage Max spectrometer. Reagents were of analytical grade and were used without further purification.
I Synthesis of Compound (1)
Potassium carbonate (5.5g, 40 mmol) and acetylacetone (2.1 mL, 20 mmol) were dissolved in 20 ml N, N-dimethylformamide and stirred for 0.5h at room temperature, CS2 (1.33 ml) was added and stirred for 1h under ice water. 1,2-dibromoethane (2.6 mL) was added dropwise and stirred for 14h at room temperature. The reaction mixture was poured into a beaker full of water (200mL), stirred until the yellow product precipitated. The precipitate was filtered off and washed with aqueous contain 95% ethanol. The crude products were recrystallized from ethanol to give the 3-(1,3-dithiolan-2-ylidene) pentane-2,4-dione (1). 3-(1,3-Dithiolan-2-ylidene)pentane-2,4-dione (1): White powder, yield 78 %, mp 165–168 oC; IR (KBr): 2919, 2407, 1628, 1193, 970 cm-1; 1H NMR (300 MHz, DMSO-d6, ppm) δ: 3.52–3.75 (t, 4H, SCH2-), 1.75 (s, 6H, -CH3); MS (ESI, m/z): 200 [M+].
II Synthesis of Curcumin Derivatives 2a-2h
Sodium (10.0 mmol) was completely dissolved in ethanol (10 mL) and stirred at room temperature, a solution of compound (1) (2.5 mmol) in ethanol, the aromatic aldehyde (6.0 mmol) was added dropwise and stirred under ice water for 4h. Then the product precipitated. The precipitate was separated by suction filtration, purified by recrystallized from industrial alcohol and dichloromethane to give the curcumin derivatives (2a-2h).
4-(1,3-Dithiolan-2-ylidene)-1,7-diphenylhepta-1,6-diene-3,5-dione (2a): Light yellow crystal, yield 79 %, mp 167–169 oC; IR (KBr): 3019(w), 1629(s), 1586(s), 1556(s), 1493(m), 1282(m), 978(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.65 (d, 2H, J=15.6Hz, -C=CH), 7.29-7.44 (m, 10H, Py-H), 6.96 (d, 2H, J=15.6Hz, -C=CH), 3.33 (s, 4H, -CH2); MS (ESI, m/z): 378.77 [M + H]+.
4-(1,3-Dithiolan-2-ylidene)-1,7-di-p-tolylhepta-1,6-diene-3,5-dione (2b): Yellow powder, yield 77%, mp 143–145 oC; IR (KBr): 3020(w), 2960(w), 2911(w), 1638(s), 1589(s), 1511(s), 1284(s), 990(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.63 (d, 2H, J=15.6Hz, -C=CH), 7.05-7.34 (m, 8H, Py-H), 6.90 (d, 2H, J = 15.6 Hz, -C=CH), 3.32 (s, 4H, -CH2), 2.27 (s, 6H, -CH3) ; MS (ESI, m/z): 406.85 [M + H]+.
4-(1,3-Dithiolan-2-ylidene)-1,7-bis(4-methoxyphenyl)hepta-1,6-diene-3,5-dione (2c): Brown powder, yield 93%; mp 151–153 oC; IR (KBr): 2969(w), 2836(w), 1622(s), 1586(s), 1511(s), 1466(m), 1287(s), 1252(s), 984(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.61 (d, 2H, J=15.6Hz, -C=CH), 6.85-7.40 (m, 8H, Py-H), 6.78 (d, 2H, J=15.6Hz, -C=CH), 3.74 (s, 6H, -OCH3), 3.31 (s, 4H, -CH2); MS (ESI, m/z): 438.79 [M + H]+.
1,7-Bis(4-(tert-butyl)phenyl)-4-(1,3-dithiolan-2-ylidene)hepta-1,6-diene-3,5-dione (2d): Yellow crystal, yield 57%; mp 154–156 oC; IR (KBr): 3062(w), 2958(m), 2863(w), 1624(s), 1589(s), 1382(s), 1412(m), 1281(s), 980(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.63 (d, 2H, J=15.6Hz, -C=CH), 7.19-7.39 (m, 8H, Py-H), 6.91 (d, 2H, J=15.6Hz, -C=CH), 3.32 (s, 4H, -CH2), 1.22 (s, 18H, -C(CH3)3); MS (ESI, m/z): 490.91 [M + H]+.
4-(1,3-Dithiolan-2-ylidene)-1,7-bis(4-fluorophenyl)hepta-1,6-diene-3,5-dione (2e): Yellow- green powder, yield 73%; mp 201–203 oC; IR (KBr): 3072(w), 3035(w), 2921(w), 1630(s), 1582(s), 1561(s), 1506(s), 1417(s), 1279(m), 982(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.67 (d, 2H, J=15.6Hz, -C=CH), 6.99-7.51 (m, 8H, Py-H), 6.92 (d, 2H, J=15.6Hz, -C=CH), 3.41 (s, 4H, -CH2); MS (ESI, m/z): 414.77 [M + H]+.
1,7-Bis(4-chlorophenyl)-4-(1,3-dithiolan-2-ylidene)hepta-1,6-diene-3,5-dione (2f): Yellow powder, yield 62%; mp 188–190 oC; IR (KBr): 3061(w), 2923(w), 1631(s), 1592(s), 1489(s), 1489(s), 1280(m), 980(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.66 (d, 2H, J=15.6Hz, -C=CH), 7.23-7.45 (m, 8H, Py-H), 6.97 (d, 2H, J=15.6Hz, -C=CH), 3.42 (s, 4H, -CH2); MS(ESI, m/z): [M + H]+.
4-(1,3-Dithiolan-2-ylidene)-1,7-di(thiophen-2-yl)hepta-1,6-diene-3,5-dione (2g): Dark yellow crystal, yield 45%; mp 159–161 oC; IR (KBr): 3098(w), 3019(w), 2918(w), 1625(s), 1571(s), 1469(s), 1278(s), 973(s) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.83 (d, 2H, J=15.2Hz, -C=CH), 7.04-7.36 (m, 6H, HeterocyclicH), 6.81 (d, 2H, J=15.3Hz, -C=CH), 3.39,(s, 4H, -CH2); MS(ESI, m/z): 390.77[M + H]+.
4-(1,3-Dithiolan-2-ylidene)-1,7-di(furan-2-yl)hepta-1,6-diene-3,5-dione (2h): Yellowish brown powder, yield 45%; mp 125–127 oC; IR (KBr): 3122(w), 3022(w), 2926(w), 1632(s), 1588(s), 1476(m), 1276(s), 992(w) cm-1; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.45 (d, 2H, J=15.6Hz, -C=CH), 7.44 (s, 2H, HeterocyclicH), 6.85 (d, 2H, J=15.3Hz, -C=CH), 6.66 (d, 2H, J=3.6Hz, HeterocyclicH), 6.45 (dd, 2H, J=1.8Hz, J=3.3Hz, HeterocyclicH), 3.38 (s, 4H, -CH2), MS(ESI, m/z): 490.91 [M + H]+.
III Procedure for Anti-Bacterial Activity
The synthesized curcumin derivatives (2a-2h) were screened for their in vitro antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Aspergillus niger. Their MICs were evaluated by using serial tube dilution methods at various concentration of 256, 128, 64, 32, 16, 8, 4, 2, 1 µg/mL [17]. The MIC, defined as the lowest concentration of the test derivative which inhibits the visible growth after 24 h, was determined visually after incubation at 37°C. Tests using DMSO as negative control were carried out in parallel. Curcumin was used as standard for antimicrobial activity.
Acknowledgment
The authors gratefully acknowledge financial support from National Natural Science Foundation of China (No. 31870328) and Hubei Key Laboratory of Pollutant Analysis and Reuse Technology (No. PA160203).
Article Info
Article Type
Research ArticlePublication history
Received: Sat 04, Jan 2020Accepted: Wed 22, Jan 2020
Published: Tue 28, Jan 2020
Copyright
© 2023 Dunjia Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JBEM.2019.01.04
Author Info
Dan Wang Dunjia Wang Heng Lyu Hengyi Du Lian Cai
Corresponding Author
Dunjia WangCollege of Chemistry and Chemical Engineering, Hubei Key Laboratory of Pollutant Analysis and Reuse Technology, Hubei Normal University, Huangshi, China
Figures & Tables
Table 1: The MIC values(µg/mL)of curcumin derivatives (2a–2h).
|
Compounds |
S. aureus |
B. subtilis |
E. coli |
A. niger |
|
2a |
64 |
64 |
64 |
32 |
|
2b |
64 |
64 |
32 |
64 |
|
2c |
32 |
64 |
64 |
32 |
|
2d |
64 |
64 |
64 |
64 |
|
2e |
16 |
8 |
16 |
8 |
|
2f |
8 |
8 |
16 |
16 |
|
2g |
8 |
8 |
8 |
8 |
|
2h |
16 |
8 |
8 |
16 |
|
Curcumin |
128 |
128 |
>256 |
>256 |
References
- Kuttan R, Bhanumathy P, Nirmala K, George MC (1985) Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett 29: 197-202. [Crossref]
- Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23: 363-398. [Crossref]
- Sharma OP (1976) Antioxidant activity of curcumin and related compounds. Biochem Pharmacol 25: 1811-1812. [Crossref]
- Srimal RC, Dhawan BN (1973) Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 25: 447-452. [Crossref]
- K Kohli, J Ali, MJ Ansari, Z Raheman (2005) Curcumin: A natural anti-inflammatory agent. Indian J Pharmacol 37: 141-147.
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R (1995) Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett 94: 79-83. [Crossref]
- Jagetia GC, Aggarwal BB (2007) “Spicing up” of the immune system by curcumin. J Clin Immunol 27: 19-35. [Crossref]
- Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F et al. (2005) Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223: 181-190. [Crossref]
- Baum L, Ng A (2004) Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis 6: 367-377. [Crossref]
- Thangapazham RL, Sharma A, Maheshwari RK (2006) Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J 8: 443-449. [Crossref]
- Ferrari E, Pignedoli F, Imbriano C, Marverti G,Basile V et al. (2011) Newly synthesized cucumin derivatives: crosstalk between chemico-physical properties and biological activity. J Med Chem 54: 8066-8077. [Crossref]
- Iranshahi M, Chini MG, Masullo M, Sahebkar A, Javidnia A et al. (2015) Can Small Chemical Modifications of Natural Pan-inhibitors Modulate the Biological Selectivity? The Case of Cucumin Prenylated Derivatives Acting as HDAC or mPGES-1 Inhibitors. J Nat Prod 78: 2867-2879.
- Anand P, Kunnumakkara AB, Newman R A, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharmaceutics 4: 807-818. [Crossref]
- Lenhart JA, Ling X, Gandhi R, Guo TL, Gerk PM et al. (2010) “Clicked” bivalent ligands containing curcumin and cholesterol as multifunctional abeta oligomerization inhibitors: design, synthesis, and biological characterization. J Med Chem 53: 6198-6209. [Crossref]
- Reid RC, Yau M-K, Singh R, Lim J, Fairlie DP (2014) J Am Chem Soc 136: 11914.
- Gibson S, McGuire R, Rees DC (1996) Principal components describing biological activities and molecular diversity of heterocyclic aromatic ring fragments. J Med Chem 39: 4065-4072. [Crossref]
- Pandey KS, Khan N (2008) Arch Pharm Chem Life Sci 341: 418.
