Journals
Systematic investigation of a new nanoscale bioactive glass on wound healing in vivo in comparison with the clinically applied 45S5 Bioglass
A B S T R A C T
Aim: Recently, bioactive glass (BG)-based inorganic biomaterials which differ from polymer wound dressings have become a promising candidate favorable for improving acute wound healing and even some chronic, non-healing wound repair. We developed a new sol–gel-derived BG (Sol-nBG) nanomaterial and evaluated its biological efficacy on wound healing based on the antibacterial and wound-healing accelerating potentials of the specific trace elements in the chemical composition of Sol-nBG.
Materials and methods: The sol-nBG powder was prepared by a facile sol–gel route which readily resulted in nanoscale particles after undergoing a low-heat calcination process. The amorphous nature of the Sol-nBG powder was confirmed by X-ray diffraction analysis, and the biologically-active ions such as calcium, silicon, zinc, and boron could be rapidly released in Tris buffer, similar to the orthopedically and dentally available 45S5 Bioglass@ (45S5 BG). In particular, the Sol-nBG and 45S5 BG particles were well tolerated by the surrounding host wound tissue without causing any chronic inflammation, and appreciably enhanced the wound healing of a deep second-degree scald.
Results and Conclusions: The results showed that the Sol-nBG significantly promoted wound healing with increased epidermal and dermal regeneration and collagen deposition in comparison with the 45S5 BG or control treatment. Therefore, it is reasonable to consider that the new Sol-nBG facilitates wound treatment and is a promising candidate for wound healing in future.
K E Y W O R D S
Nanoscale bioactive glass, 45s5 Bioglass@, Boron, Zinc, wound healing
I N T R O D U C T I O N
In various wound operations, the purpose of dressing the wound is to promote wound healing by creating an optimal healing environment, providing pain relief and protection from trauma and infection. Biological dressings may serve as scaffolds for delivery of growth factors and/or cells to wounds and modulate both the physical and molecular environment of the wound [1]. By simultaneously maximizing the patient’s nutritional status and providing meticulous wound care, most wounds will heal appropriately [2]. However, chronic, non-healing wounds involve progressive tissue loss and bacterial colonization, particularly in venous stasis, diabetic ulcers, and bed sores, and thus provide a challenge to the wound-care research community [3-6].
It is well established that calcium is an important factor in wound healing of skin and it is assumed that it is required for the migration of epidermal cells. Clinically, the direct topical application of calcium to various wounds via calcium alginate dressings has been shown to be beneficial [7]. Moreover, bacterial invasion may make the wound unsuitable for skin regeneration due to growth of the bacterial population. Thus, the goal when creating a wound dressing is to produce an ideal material with high antimicrobial activity which can also form a good barrier. Over the past decade, research has shown that the sustained release of some biologically-essential inorganic ions (such as zinc (Zn), boron (B), silicon (Si) and calcium (Ca) from bioactive glasses (BGs) can suppress microorganisms, stimulate collagen production, and/or accelerate the wound healing process [8-11]. In particular, some silicate-based or borate BG with specific chemical compositions are able to reduce the inflammatory response and wound exudate, and have certain antibacterial and hemostatic potential, all of which have an appreciable therapeutic effect on a wound [12, 13].For instance, Wilson et al. first demonstrated that soft connective tissues were able to form a bond to 45S5 Bioglass® (45S5 BG) and they established the safety of the use of particulate forms in soft tissue if the interface was immobile[14]. Lin et al. compared the effect of sol–gel-derived 58S and melt-derived 45S5 BG on cutaneous wound healing in full thickness excision wounds in both normal and diabetic rats [15]. It has been shown that the sol–gel 58S BG healed wounds more quickly and efficiently than 45S5 BG, and in particular it has been demonstrated that 58S can accelerate the recovery of skin wounds in both normal and diabetes-impaired healing models. More recent studies demonstrated the beneficial effects of borate-containing 13–93 BGs in promoting angiogenesis, which is critical to the healing of soft tissue wounds [16, 17]. These findings greatly expanded the vision of using BG materials both to stimulate soft tissue healing and to synergistically enhance antibacterial effects. Thus, the development of BG-loaded porous wound dressings with antimicrobial activity is highly desired.
Beyond promoting soft tissue healing, BGs have also been proven to be beneficial for antibacterial action in the wound environment. Nano-scale BGs prepared by the sol–gel method was found to perform better as they have a larger specific surface area [18, 19]. In our previous study, the nanoscale silicate-borate BGs including 20.0% B2O3 and 1.0% ZnO were prepared by a sol–gel process and their antibacterial effects and biocompatibility were investigated systematically [20]. In this study, we developed a new B2O3-rich, ZnO-containing sol–gel-derived nanoscale BG (Sol-nBG; see Table. 1) based on the chemical composition of the orthopedically and dentally available 45S5 BG. Furthermore, in vivo evaluations and comparison of these two BGs are thoroughly discussed for their bioactive effects on the healing of deep second-degree scald wounds in a rat model.
Material and Methods
Materials and Chemicals
Medical vaselinum, tetraethyl orthosilicate (TEOS; 99.99%), triethyl phosphite (P(OEt)3; 99.50%), and inorganic salts were purchased from Sinopharm Chemical Reagent Co., Shanghai, China. All the chemicals were of analytical grade and were used directly without further purification.
Synthesis of BGs
The Sol-nBG comprising 33.0 SiO2, 22.5 CaO, 12.0 B2O3, 6.0 P2O5, 2.0 ZnO, and 6.0 Na2O (wt%) was prepared by a sol–gel process based on the 45S5 BG composition (see Table 1). In brief, HCl, H3BO3, TEOS, and P(OEt)3 were added into an ethanol-water mixture and stirred for 20 min. The nitrates of calcium, sodium, and zinc were then added into deionized water (Di water) under magnetic stirring for 20 min. The solutions were mixed and subsequently aged at 80°C for 36 h then calcined at 650°C for 90 min. The as-obtained Sol-nBG powder was ball milled for 2 h to obtain a smaller particle size then characterized by scanning electron microscopy (SEM; JSM-6700F, Jeol Ltd, Tokyo, Japan). The particle size distribution data were collected by dynamic light scattering (DLS) in a Mastersizer 2000 analyzer (Malvern Instruments Ltd., Malvern, UK).
Inorganic Ion Release Test in vitro
The inorganic ion release was investigated for the Sol-nBG and 45S5 BG (2.0 g) by immersing in 50 mL Tris buffer (0.05 M) with an initial pH of 7.25 at physiological temperature in vitro. After immersing for different time intervals, changes in the pH of the aqueous medium were measured first; then 0.5 mL of supernatant was diluted in 5% HCl solution, and 0.5 mL aliquots of fresh buffer were added into the buffers to maintain a constant volume of solution. The inorganic mineral elements Ca, Zn, B, and Si in the supernatant were determined using inductively-coupled plasma spectrometry (ICP; IRIS INTREPID II XSP, Thermo Fisher Scientific, Waltham, MA, USA) analysis.
Wound Healing Assessment
The area of each wound was measured using the transparent membrane tracing method. The percentage of wound closure was calculated as follows:
(Area of original wound - area of actual wound/area of original wound) × 100%.
Histochemistry and Immunohistochemistry
The rats were sacrificed at 7 and 14 d after beginning the different treatments, and the wound and surrounding skin were harvested by punch biopsy. Freshly-harvested samples were immediately removed, immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 24 h, and dehydrated with dH2O for 30 minutes, then rinsed in 75% ethanol for 1 h, 85% ethanol for 1 h, 95% ethanol for 1 h twice, 100% ethanol for 1 h twice, absolute xylene for 20 min twice, and then immersed in paraffin at 58–60°C for 3 h. After the tissue was paraffin embedded, 4 μm coronal serial sections were cut (Microm HM-340E microtome, Microm, Walldorf, Germany) for hematoxylin and eosin staining (HE).
The specimens were stained using the EnVision™ two-step strategy and high-temperature antigen retrieval (pressure cooker, Supor Co, China). Four-micron-thick paraffin-embedded sections were deparaffinized twice, for 10 minutes each time, in 100% xylene, and then hydrated with 100% ethanol for 5 min twice, 95% ethanol for 3 min, and 80% ethanol for 5 min. After soaking for 2-5 min in distilled water, the slides were put into the pressure cooker containing 1 L of boiling sodium citrate buffer (pH 9.0) and heated under pressure. Two minutes after steaming, the pressure cooker was removed from the heat source and cooled down to room temperature with tap water. The container was opened, and the slides were rinsed for 2 × 3 min with PBS. Afterwards, the slides were incubated with 3% hydrogen peroxide for 10 minutes followed by another 2 × 3-min rinses in PBS. Then the primary antibody [rabbit anti rat CD31, (EPR17259) Abcam, Cambridge, UK; ab182981, 1:500) was applied for 60 minutes in a humidified chamber at 37°C. After 2 × 3-min rinses in PBS, the slides were incubated with horseradish peroxidase (HRP) polymer for 30 min at 37°C, then color was developed using DAB chromogen, and the slides were observed and examined for color change under a light microscope. This was followed by counterstaining with hematoxylin for 1 min, then rinsing in tap water for 1 min.
For Masson’s trichrome staining, the tissue sections were deparaffinized and rehydrated, then stained according to the instructions provided with the Masson’s trichrome Staining Kit (Maixin-Biotech, Fuzhou, China). Briefly, the sections were deparaffinized and rehydrated through xylene and ethanol, and stained in Weigert’s iron hematoxylin working solution, followed by staining in Biebrich scarlet acid fuchsin solution. The sections were then differentiated in phosphotungstic acid solution and transferred directly (without rinsing) into Aniline blue solution. After rinsing, the sections were differentiated in 1% acetic acid solution, followed by dehydration in 95% ethyl alcohol and absolute ethyl alcohol. This method allows for the collagen to be stained blue.
Statistical Analysis
Results are expressed as mean ± standard deviation (mean ± SD). Statistical significance was tested by one-way ANOVA, and differences of P < 0.05 were considered to be statistically significant.
Figures & Tables
Table 1. Chemical composition in the BG powders (wt%)
Figure 1: XRD patterns (A, B) and TEM micrographs (C, D) of the newly synthesized Sol-nBG (A,C) and clinically available 45S5 (B,D) BG powders
Figure 2: TG-DTA analysis of the newly synthesized Sol-nBG (A) and clinically available 45S5 (B) BG powders.
Figure 3: Changes in Ca and Si (A, B) and B and Zn (C) concentrations of the Tris buffer media during immersing the clinically available 45S5 BG (A) and newly synthesized Sol-nBG (B,C) powders.
Figure 4: (A) Outward appearance of the wound during healing process covered with and without BG-based and (B) wound closure percentage in all groups. *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5
Figure 5: HE staining examination of skin tissues and epidermal thickness. Representative images showing HE staining of skin tissues (A) and pathological examination of epithelium (B) on 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). (C) Aanalysis of epidermal thickness on 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5.
Figure 6: IHC CD31 staining and capillary density. (A) Representative images showing CD31 staining (yellow) and (B) Capillary density on 7 days and 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5.
Figure 7: Masson staining for collagen fibers. Representative trichrome-stained images on 7 days and 14 days after wound treatment with Sol-nBG, 45S5 and the control
Results and Discussion
Characterization of Morphological Composition
The sol–gel method is more versatile for the preparation of nanoscale BG powders in comparison to high-temperature calcination treatment. The X-ray diffraction patterns Figure 1(A, C) demonstrated that both the Sol-nBG and 45S5 BG powders were amorphous in nature after undergoing either a low thermal or a high thermal preparation process. In particular, the SEM images shown in Figure 1(B, D) confirmed the ultrafine Sol-nBG and 45S5 BG powders prepared by a sol–gel process in this study and by the conventional high-temperature melting method respectively. The former consisted of nanoscale particles with some degree of aggregation while the latter comprised significantly larger particle sizes of several microns. It should be mentioned that the measured composition of Sol-nBG powders was 33.7 SiO2, 22.3 CaO, 11.6 B2O3, 5.7 P2O5, 2.1 ZnO, and 24.6 Na2O (wt%), similar to the theoretical values shown in Table 1. These primary results suggested that the Sol-nBG was a B2O3-rich, ZnO-doped new BG nanomaterial, in which much of the SiO2 (12.0%) and a minor amount of the CaO (2.0%) were substituted by B2O3 and ZnO, respectively. In this regard, the Sol-nBG might be considered the derivate of the clinically-available 45S5 BG according to their chemical compositions. In order to endow the new BG nanomaterial with multifunctional properties, the Sol-nBG with the addition of appropriate amounts of B2O3 and ZnO is highly interesting with regard to both wound healing and antibacterial aspects.
Figure 2 shows the TG-DTA curves for the NCS-xB and 45S5 BGs. It shows a glass transition temperature (Tg, endothermic effect) in the range of 480−560°C and crystallization temperature (Tc, exothermic effect) at 647°C for the 45S5 BG. With the addition of B2O3 and ZnO, both the glass transition and crystallization temperatures shifted upwards for the Sol-nBG. In contrast, the DTA performed on the Sol-nBG revealed an appreciable endothermic melting peak (Tm; ~972°C) in comparison with that of 45S5 BG (~1190°C) reported previously. Moreover, as the heating process proceeded, only a minor weight loss (< 10%) occurred at 30−600°C on TG curves. These primary results suggest that the sol–gel-derived Sol-nBG with addition of appreciable amounts of B2O3 and ZnO (12% and 2%, respectively) mainly works at below 900°C and specific heat treatment close to the onset of the melting temperature should be avoided for all practical aspects.
Ion Release Behavior
Examination of the ion release behavior in Tris buffer over time may provide some insight into the biodegradation of BGs. Figure 3 shows the changes of Ca, Si, B, and Zn concentrations in Tris buffer as a function of soaking time. The changes in Ca and Si concentration were consistent with particle size (or specific surface area) of the BGs (Figure 3A, B). Appreciable differences in Ca concentration were observed for the Sol-nBG and 45S5 BG within the initial time stage (2 h) but were followed by a similar trend in ion concentrations. As for the Si concentration during immersion of the two types of BGs, it increased rapidly in Tris buffer within 2 h and continued to increase very slowly. With Sol-nBG, the B concentration rapidly increased but the concentration of Zn experienced an increase but then stabilized at a certain value within 72 h (Figure 3C). Obviously, the increase in Si and Ca concentrations in the initial stage were attributed to the dissolution of non-crystalline particles and the ion release from BGs, respectively. Moreover, it is important to note that the pH trend for the Sol-nBG during soaking in Tris buffer also showed a rapid increase from 7.25 to 8.84 within the initial 2 h, and then increased up to 9.24 at 72 h (data not shown). The lower specific surface area of the 45S5 BG microparticles favored slower ion leaching compared to that for the new composition, and the pH value was between 7.76 and 7.98 throughout the process for this clinically-available BG.
Sol-nBG Enhances Wound Healing
Wound healing and the percentages of wound closure at different times are shown in Figure 4. The results show that the wound closure percentage in the Sol-nBG-treated rats was significantly greater than that in the other groups, suggesting that Sol-nBG accelerated wound closure compared with 45S5 BG and control (P < 0.05).
Histopathological Evaluations
HE staining revealed only a few scattered lymphocytes in the ulcer or repair area of the three groups, and lack of obvious or extensive infiltration of inflammatory cells or inflammatory lesions. The proliferation of epithelial cells around the ulcer gradually increased over the 7 d after treatment, and gradually spread to the ulcer surface, slowly covering the ulcer granulation tissue. At the same time, the subcutaneous appendages (hair follicles, sweat glands and sebaceous glands) began to slowly proliferate and migrate around the periphery. The number of cell layers or epithelial layer thickness was the highest for Sol-nBG at 7 d after starting treatment, followed by 45S5 BG, and lowest in controls. However epithelial cell proliferation was the thinnest at 14 d after starting treatment and was most significant in the Sol-nBG group. The epithelial cell layer became thinner (Figure 5A, C), and the new epithelial cells almost covered the entire ulcer surface, suggesting that epithelial proliferation reduced in the Sol-nBG-treated wound after 14 d of treatment. Compared with 45S5 BG and control, Sol-nBG-treated wounds demonstrated more orderly arranged layers of epithelial cells, as well as increased numbers of skin appendages (hair follicles, sweat glands and sebaceous glands, Figure 5A), indicating a more mature epithelium. These results indicate that Sol-nBG accelerates epidermal regeneration. In addition, after 7 d of treatment, in both the 45S5 BG and Sol-nBG-treated wounds, but especially Sol-nBG, many fibroblasts and large amounts of collagen were observed within the repaired granulation tissue. The number of new blood vessels was further decreased by 14 d of treatment, along with a significantly increased number of collagen fibers in the 45S5- and Sol-nBG-treated wounds, but especially in the Sol-nBG group. However, by 14 d of treatment, neovascularization remained high in the control group. These results show that Sol-nBG-treated wounds repair faster than those treated with 45S5 BG or control.
Effect of Sol-nBG on Neovascularization
To further clarify the neovascularization status, we performed IHC staining of CD31. When observed by light microscopy, endothelial cells of new blood vessels appeared brownish yellow, which was used to distinguish the blood vessels and to count the number of new blood vessels per unit area (Figure 6A). The results showed that the blood vessel density of Sol-nBG-treated wounds was increased after 7 d of treatment compared with the 45S5 and control groups (P < 0.05). However, the number of blood vessels in the Sol-nBG-treated wounds was reduced to a level significantly lower than those of the control and 45S5 groups (P < 0.05). After14 d of treatment, a large number of new blood vessels were still distributed within the granulation tissue of the control, and the number was significantly higher than that of Sol-nBG group (P < 0.05; Figure 6). The results showed that Sol-nBG can promote blood vessel proliferation and early-stage wound healing.
Collagen Deposition
Masson’s trichrome staining was performed to evaluate the number of collagen fibers deposited. As shown in Figure 7, collagen fibers tended to increase over time in all three conditions. By 7 d after the start of treatment, there were fewer collagen fibers with a cotton-floss-like distribution in the control. After 14 d of treatment, the arrangement of collagen was relatively concentrated and irregular, and was wound into a spiral with extensive neovascularization. In the 45S5 and Sol-nBG groups, the numbers of collagen fibers increased significantly within 7 d of treatment and were visible in bands or clump-like shapes at an early stage. By 14 d the collagen fibers in the Sol-nBG group were relatively loose and slender, arranged in an orderly manner and basically parallel to the skin, with collagen morphology and distribution close to those of normal tissue.
BGs are a type of reactive non-crystalline inorganic biomaterial with appreciable biocompatibility and bioactivity, making them suitable for use as implant devices in the human body for bone repair and even soft tissue wound healing [21, 24]. The functions of BGs are the result of the controlled release of ions [25, 26]. 45S5Bioglass@, an important bioactive glass, was first developed by Professor Hench in 1971 to help regenerate bone and was composed of 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O and 6.0 wt% P2O5. Some studies have demonstrated that 45S5 is clinically available to promote the wound healing of soft tissues and has potential antibacterial potential as well as angiogenic properties [27-32]. In addition, some early pioneering reports investigating novel sol–gel-derived BGs have indicated that they have better biodegradability and bioactivity than traditional melt-derived BGs. Sol–gel derived BGs (sol–gel BGs) have an inherent nanoporosity and larger surface area, which may help improve cellular responses and enhance tissue healing and repair [33, 34]. Lin et al. found that the sol–gel BGs can induce a shorter wound healing time in rat models, compared to melt-derived BGs [35]. Here, the Sol-nBG derived from 45S5 BG was synthesized as a model to study its potential application for wound dressing. Because the Sol-nBG particles were in the size range of 30–450 nm, the surface area was larger than 45S5 which has a relatively large size (2–10 μm). As a result, our study showed that the Sol-nBG could significantly accelerate skin wound healing compared to the well-known clinically-available 45S5 BG.
Many studies have also investigated the effects of ion release from BGs [36]. Generally, BGs mainly consist of calcium, phosphate, silicon and sodium. Among these ions, Si is considered to play a crucial role in regulating cell behaviors because of the similar concentrations of calcium and phosphate ions between BG extracts and normal control medium [8, 9, 37]. In recent years, various ions such as silver, boron, copper, zinc and its chemical compound, have been added to new biomaterials in order to achieve bioactivity in living organisms [38-40].Some researchers reported that borate BGs have a lower chemical durability, and hence a faster dissolution rate for dermal repair. In our current study, a new borate Sol-nBG (containing 12.0% B2O3 and 2.0% ZnO) was fabricated and showed accelerated wound healing in an excision wound model in rats, which was consistent with previous reports. In addition, ZnO-doped bioglass has also been applied to control the microstructure of lyophilized porous biocomposites. The bioactivity of ZnO-doped bioglass depends on the properties of zinc ions which exhibit bactericidal, immune-stimulating, and tissue-regenerating functions in the organism [40]. Our study demonstrated that Sol-nBG with 2.0% ZnO improved the rate of skin regeneration in rats, compared to 45S5 BG free of zinc in its composition.
Zinc is an essential micronutrient in the human body and a cofactor of more than 300 types of enzymes in humans [41]. Zinc binds to target proteins to exert functions including regulation of gene transcription, DNA repair, apoptosis, extracellular matrix regulation and anti-oxidative stress in the human body [42]. Zinc content is relatively high in the skin (approximately 5% of the zinc in the body), is particularly important for various skin functional adjustments and plays an important role in wound healing [43]. Studies have shown that zinc-deficient wounds demonstrate reduced epithelial regeneration and less collagen content synthesized by wound fibroblasts, as well as delayed wound healing. Zinc supplementation stimulates proliferation of granulation tissue, capillaries, epithelial cells and fibroblasts, promoting collagen synthesis and accelerating wound healing. Studies have confirmed that after scalding, zinc redistributes in the body, leading to decreased blood zinc and increased zinc in the wound. However, fibroblast proliferation and collagen synthesis during wound healing increase the demand for zinc in the wound area, and the increased local zinc concentration still cannot meet the needs of wound repair. Zinc content in the local skin of a wound is lower than normal. Local application of zinc at wound sites can supplement zinc deficiency in local tissue, which is conducive to cell proliferation and collagen synthesis, and promotes wound healing. Studies have shown that local zinc application to wounds can promote hemostasis and platelet activation. The promotion of platelet activation is mediated through protein kinase C (PKC)-related platelet tyrosine phosphorylation and activation of platelet release of various cytokines, such as CXCL4, CXCL5, CXCL7, CXCL8, and CXCL12, which can recruit natural immune cells to migrate and penetrate into damaged wounds [44]. These innate immune cells can promote wound healing by eliminating cell debris and microbes in the wound. Studies found that zinc supplementation at the wound surface can upregulate the number of regulatory T cells (Tregs) [45], which help to eliminate inflammation, promote epithelial regeneration and wound contraction, and accelerate wound healing. Collagen/extracellular matrix (ECM) deposition is critical to the wound healing process. One of the major regulators of ECM deposition is transforming growth factor (TGF)/SMAD. Zinc is an important cofactor of SMAD signaling. Consequently, local zinc supplementation on the wound surface can promote the deposition of collagen/ECM and accelerate wound healing. Studies have shown that zinc supplementation at a local wound site not only promotes epithelial cell regeneration of wound tissue, but also promotes keratinocyte migration and epidermal re-epithelization [46]. Local wound zinc supplementation can also promote the migration of vascular endothelial cells to the wound and their proliferation and promote the formation of new blood vessels in the wound, so as to provide enough oxygen and nutrients for cell growth. Zinc is also an anti-oxidant and anti-stress trace element. Studies have shown that zinc deficiency will increase oxidative stress at wound sites, and delay healing. A large proportion of metallothionein (MT) in cells (nearly 20%) binds to zinc, which can also be released according to the physiological needs of the micro-environment. MTs are sensitive antioxidant proteins, so zinc plays an important role in the regulation of antioxidant stress. Meanwhile cell membrane repair plays an important role in wound repair. RIM and TRIM have great potential in wound repair by repairing cell membranes and are an important part of wound healing, promoting wound healing and reducing scar formation. Zinc can modulate the expression and function of TRIM protein in the wound-healing process [47]. In this study, zinc oxide nanoparticle technology was used to promote the absorption of zinc ions. Nanomaterials can promote highly-efficient penetration of zinc deep into tissues and cells, increasing the zinc content in wound tissues where it exerts its physiological functions of wound repair.
As a common clinical disinfectant, boric acid solution is weakly acidic with low cytotoxicity. It has been reported that treatment of wounds with boric acid solution can promote wound healing by increasing granulation tissue formation and collagen synthesis [48]. The mechanism may be that boric acid can create a weakly-acidic environment at the wound, which can effectively suppress microbial growth and prevent local wound infection, thereby promoting wound healing. Activity of intracellular enzymes, synthesis of macromolecules, transport of metabolites, and cell cycle progression all depend on an appropriate pH inside and outside the cells. A study of refractory wounds found that the pH of most refractory wounds is alkaline. Boric acid changes the basic conditions of the wound, adjusts the pH of the wound environment, and promotes wound healing. At the same time, boric acid stimulates the expression of TGF-β1 in the wound, which can stimulate collagen synthesis and promote wound healing.
Conclusions
In summary, we have successfully developed a new 45S5-derived BG by adding B2O3 and ZnO in a sol–gel route. The low-temperature calcination treatment could be favorable for obtaining nanoscale non-crystalline powders. Based upon our in vivo results, we can conclude that such new sol–gel derived BGs containing the selected specific biologically essential ions such as boron and zinc had smaller diameters and thus enhanced biodegradability and bioactivity compared to the clinically-available melt-derived 45S5 BG. Consequently, our novel sol–gel derived BG is expected to provide a potential therapeutic application for skin wound dressing.
Acknowledgements
This work was financially supported by project grant funding from Health and Family Planning Commission of Zhejiang Province (No. 2016148438), National Natural Science Foundation of China (81601881, 81772311), the Science and Technology Department of Zhejiang Province Foundation (LGF18E020001; 2016C33055; 2017C37134).
Article Info
Article Type
Research ArticlePublication history
Received: Tue 20, Mar 2018Accepted: Thu 05, Apr 2018
Published: Tue 10, Apr 2018
Copyright
© 2023 Zhongru Gou. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2018.10.001
Author Info
Jianhua Shen Weizhong Gu Xianyan Yang Yunling Li Zhongru Gou Zhouwen Jin
Corresponding Author
Zhongru GouZhejiang-California International NanoSystems Institute, Zhejiang University, Hangzhou, 310058, China
Figures & Tables
Table 1. Chemical composition in the BG powders (wt%)




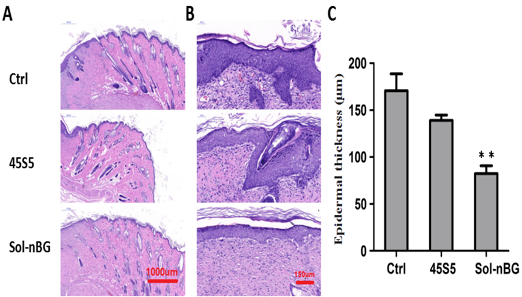


Figure legends
Fig. 1: XRD patterns (A, B) and TEM micrographs (C, D) of the newly synthesized Sol-nBG (A,C) and clinically available 45S5 (B,D) BG powders
Fig. 2: TG-DTA analysis of the newly synthesized Sol-nBG (A) and clinically available 45S5 (B) BG powders.
Fig. 3: Changes in Ca and Si (A, B) and B and Zn (C) concentrations of the Tris buffer media during immersing the clinically available 45S5 BG (A) and newly synthesized Sol-nBG (B,C) powders.
Fig. 4: (A) Outward appearance of the wound during healing process covered with and without BG-based and (B) wound closure percentage in all groups. *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5
Fig. 5: HE staining examination of skin tissues and epidermal thickness. Representative images showing HE staining of skin tissues (A) and pathological examination of epithelium (B) on 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). (C) Aanalysis of epidermal thickness on 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5.
Fig. 6: IHC CD31 staining and capillary density. (A) Representative images showing CD31 staining (yellow) and (B) Capillary density on 7 days and 14 days after wound treatment with Sol-nBG, 45S5 and the control (Ctrl). *represents P < 0.05, and **represents P < 0.01 as compared the data with control and 45S5.
Fig. 7: Masson staining for collagen fibers. Representative trichrome-stained images on 7 days and 14 days after wound treatment with Sol-nBG, 45S5 and the control
References
1. Zielins ER, Atashroo DA, Maan ZN, Duscher D, Walmsley GG et al. (2014) Wound healing: an update. Regen Med 9: 817-830. [Crossref]
2. Das S, Baker AB (2016) Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng Biotechnol 4: 82. [Crossref]
3. Gould L, Abadir P, Brem H, Carter M, Conner-kerr T, et al. (2015) Chronic Wound Repair and Healing in older Adults: Current Status and Future Research. Wound Repair Regen 23: 1-13. [Crossref]
4. Guo S, DiPietro LA (2010) Factors Affecting Wound Healing. J Dent Res 89: 219-229. [Crossref]
5. Turner NJ, Badylak SF (2015) The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv Wound Care (New Rochelle) 4: 490-500. [Crossref]
6. Lansdown AB (2002) Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen 10: 271-285. [Crossref]
7. Barnett SE, Varley SJ (1987) The effects of calcium alginate on wound healing. Ann R Coll Surg Engl 69: 153-155. [Crossref]
8. Xie W, Chen X, Miao G, Tang J, Fu X (2016) Regulation of cellular behaviors of fibroblasts related to wound healing by sol–gel derived bioactive glass particles. J Biomed Mater Res A 104: 2420-2429. [Crossref]
9. Li H, He J, Yu H, Green CR, Chang J (2016) Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 84: 64-75. [Crossref]
10. Demirci S, Doğan A, Aydın S, Dülger EÇ, Şahin F (2016) Boron promotes streptozotocin-induced diabetic wound healing: roles in cell proliferation and migration, growth factor expression, and inflammation. Mol Cell Biochem 417: 119-133. [Crossref]
11. Kaya S, Cresswell M, Boccaccini AR (2018) Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater Sci Eng C 83: 99-107.
12. Miguez-Pacheco V, Hench LL, Boccaccini AR (2015) Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater 13: 1-15. [Crossref]
13. Chen S, Yang Q, Brow RK, Liu K, Brow KA et al. (2017) In vitro stimulation of vascular endothelial growth factor by borate-based glass fibers under dynamic flow conditions. Mater Sci Eng C Mater Biol Appl 73: 447-455. [Crossref]
14. Wilson J, Pigott GH, Schoen FJ et al. (1981) J Biomed Maters Res 15: 805-815.
15. Lin C1, Mao C, Zhang J, Li Y, Chen X (2012) Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed Mater 7: 045017. [Crossref]
16. Zhou J, Wang H, Zhao S, Zhou N, Li L, et al. (2016) In vivo and in vitro studies of borate-based glass micro-fibers for dermal repairing. Mater Sci Eng C 60: 437-445. [Crossref]
17. Yang Q, Chen S, Shi H, Xiao H3, Ma Y (2015) In vitro study of improved wound-healing effect of bioactive borate-based glass nano-/micro-fibers. Mater Sci Eng C Mater Biol Appl 55: 105-117. [Crossref]
18. MacEwan MR, MacEwan S, Kovacs TR, Batts J (2017) What Makes the Optimal Wound Healing Material? A Review of Current Science and Introduction of a Synthetic Nanofabricated Wound Care Scaffold. Cureus 9: 1736. [Crossref]
19. Wang C, Zhu F, Cui Y, Ren H, Xie Y, et al. (2017) An easy-to-use wound dressing gel gelatin-bioactive nanoparticle and its preliminary in vivo study. J Mater Sci Mater Med 28: 10. [Crossref]
20. Ma W, Yang X, Ma L, et al. (2014) Fabrication of bioactive glasses-introduced nanofibrous membranes with multifunctions potentially for wound dressing. RSC Adv 4: 60114-60122.
21. Gillette RL, Swaim SF, Sartin EA, Bradley DM, Coolman SL (2001) Effects of a bioactive glass on healing of closed skin wounds in dogs. Am J Vet Res 62: 1149-1153. [Crossref]
22. Julian RJ, Delia SB, Leena H, et al. (2016) Bioglass and Bioactive Glasses and Their Impact on Health care. International Journal of Applied Glass Science 7: 423-434.
23. Hench LL, Thompson I (2010) Twenty-first century challenges for biomaterials. J R Soc Interface 7: S379–S391. [Crossref]
24. Makhni MC1, Caldwell JM1, Saifi C2, Fischer CR2, Lehman RA, et al. (2016) Tissue engineering advances in spine surgery. Regen Med 11: 211-22. [Crossref]
25. Mishra R, Bishop T, Valerio IL, Fisher JP, Dean D (2016) The potential impact of bone tissue engineering in the clinic. Regen Med 11: 571-587. [Crossref]
26. HoppeA, Guldal, NS, Boccaccini, AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32: 2757-2774. [Crossref]
27. Moosvi SR, Day RM (2009) Bioactive glass modulation of intestinal epithelial cell restitution. Acta Biomater 5: 76-83. [Crossref]
28. Allan I, Newman H, Wilson M (2001) Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 22: 1683-1687. [Crossref]
29. Waltimo T, Brunner TJ, Vollenweider M, Stark WJ, Zehnder M (2007) Antimicrobial effect of nanometric bioactive glass 45S5. J Dent Res 86: 754-757. [Crossref]
30. Waltimo T, Mohn D, Paqué F, Brunner TJ, Stark WJ, et al. (2009) Fine tuning of bioactive glass for root canal disinfection. J Dent Res 88: 235-238. [Crossref]
31. Lin C, Mao C, Zhang J, Li Y, Chen X (2012) Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed Mater 7: 045017. [Crossref]
32. Gorustovich AA, Roether JA, Boccaccini AR (2010) Effect of bioactive glasses on angiogenesis: A Review of in vitro and in vivo evidences. Tissue Eng Part B: Rev 16: 199-207. [Crossref]
33. Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, et al. (2011) Bioactive glass in tissue engineering. Acta Biomater 7: 2355-2373. [Crossref]
34. Li Y, Hu Q, Miao G, Zhang Q, Yuan B, et al. (2016) Size-Dependent Mechanism of Intracellular Localization and Cytotoxicity of Mono-Disperse Spherical Mesoporous Nano- and Micron-Bioactive Glass Particles. J Biomed Nanotechnol 12: 863-877. [Crossref]
35. Lin C, Mao C, Zhang J, Li Y, Chen X (2012) Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed Mater 7: 045017. [Crossref]
36. Hoppe A, Guldal NS, Boccaccini AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32: 2757-2774. [Crossref]
37. Li H, Chang J (2013) Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater 9: 5379-5389. [Crossref]
38. Zi-Wei L, Li CW, Wang Q, Shi SJ, Hu M, et al. (2017) The Cellular and Molecular Mechanisms Underlying Silver Nanoparticle/Chitosan Oligosaccharide/Poly (vinyl alcohol) Nanofiber-Mediated Wound Healing. J Biomed Nanotechnol 13: 17-34. [Crossref]
39. Barlet M, Kerrache A, Delaye JM et al. (2013) SiO2-Na2O–B2O3 density: A comparison of experiments, simulations, and theory. Non-Cryst. Solids 382: 32-44.
40. Lidia C, Monika B, Zbigniew J et al. (2017) Controlling the microstructure of lyophilized porous biocomposites by the addition of ZnO-doped bioglass. International Journal of Applied Ceramic Technology 14: 1107-1116.
41. Livingstone C (2015) Zinc: Physiology, deficiency, and parenteral nutrition. Nutr Clin Pract 30: 371-382(2015). [Crossref]
42. Lin PH, Sermersheim M, Li H, Lee PHU, Steinberg SM, et al. (2017) Zinc in Wound Healing Modulation. Nutrients 10: pii: E16. [Crossref]
43. Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS (2007) Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen 15: 2-16. [Crossref]
44. Taylor KA, Pugh N (2016) The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics 8: 144-155. [Crossref]
45. Rosenkranz E, Metz CH, Maywald M, Hilgers RD, Weßels I, et al. (2016) Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol Nutr Food Res 60: 661-671. [Crossref]
46. Agren MS, Chvapil M, Franzén L (1991) Enhancement of re-epithelialization with topical zinc oxide in porcine partial-thickness wounds. J Surg Res 50: 101-105. [Crossref]
48. Cai C, Lin P, Zhu H, Ko JK, Hwang M, et al. (2015) Zinc Binding to MG53 Protein Facilitates Repair of Injury to Cell Membranes. J Biol Chem 290: 13830-13839. [Crossref]
49. Nzietchueng RM, Dousset B, Franck P, Benderdour M, Nabet P et al (2002) Mechanisms implicated in the effects of boron on wound healing. J Trace Med BioL 16: 239-244. [Crossref]
