The ADeViD Study: Alzheimer’s Dementia and Vitamin D Study
A B S T R A C T
Introduction: Aging is associated with a large increase in the prevalence of hypovitaminosis D. 25-Hydroxyvitamin D, 25(OH)D, is the best indicator for vitamin D status. Its possible role in the pathogenesis of Alzheimer’s disease (AD), the leading cause of dementia in the elderly, is particularly important. The aim of the present study was to examine the association between 25-hydroxyvitamin D (25(OH)D) and cognitive functions in a group of Italian elderly patients affected with AD.
Methods: We studied the relationship between 25(OH)D and cognitive functions assessed by MMSE (Mini Mental State Examination) in 150 consecutive elderly patients (F 76 %, age 78,66+ 6,05 years old) attending our Geriatric ambulatory for cognitive disorders with diagnosis of AD.
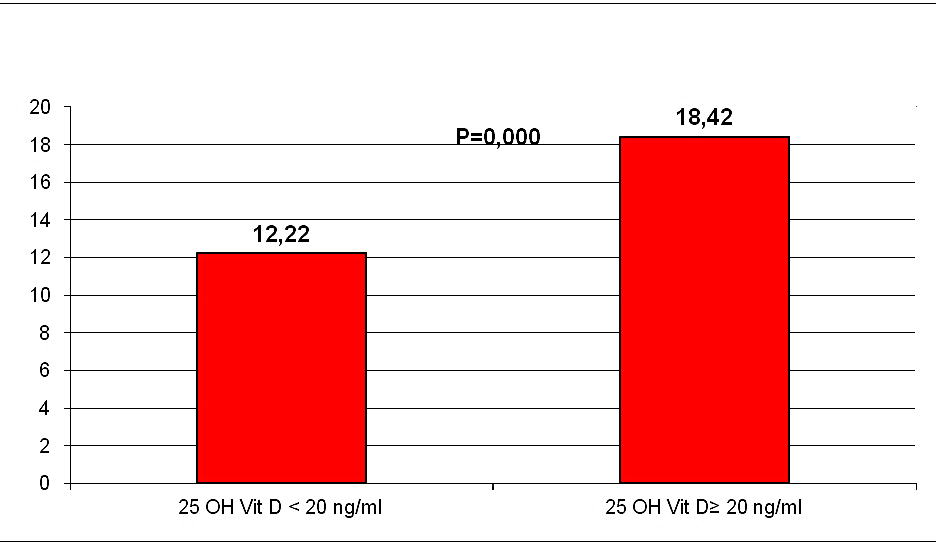
Results: In our sample hypovitaminosis D was present in 100% of the screened patients; 111 patients (74%) had 25(OH)D serum levels inferior to 20 ng/ml; 39 (26%) patients had serum levels included between 20 and 30 ng/ml. After adjustment for age, gender, systolic blood pressure, education, cardiovascular diseases and antihypertensive treatment, a significant relationship was observed between 25(OH)D and cognitive status. MMSE appeared significantly higher in subjects with 25(OH)D serum levels ≥ 20 ng/ml than in those with 25(OH)D < 20 ng/ml (18,42+4,33 vs 12,22+4,44; p=0,000).
Conclusion: Our results showed a relationship between 25(OH)D and cognitive impairment in patients with AD, suggesting that 25(OH)D could be involved in the onset of dementia.
Keywords
Hypovitaminosis D, elderly, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is caracterized by a progressive decline of cognitive performance with a detrimental impact on social activies [1-3]. A restricted ultraviolet ligh exposure, low vitamin D intake and a decreased skin synthesis capacity may be related to the development of vitamin D deficiency in ageing polpulation. Hypovitaminosis D is commonly observed in elderly popolation. Its possible role in the pathogenesis of Alzheimer’s Disease is particularly important, as AD remains a pubblic health concern with no current efficient treatment.Vitamin D is a neurosteroid hormone which crosses the blood-brain barrier and binds to vitamin receptors (VDR) present in neurons and glial cells of the central nervous system including the hippocampus, the hypothalamis, the cortex and the subcortex [4-6]. 1-25 dihydroxyvitamin D, that is the active form of vitamin D, regulates the intra-neuronal calcium homeostasis via the regulation of voltage-dependent calcium channels, thus preventing necrosis and has also showen neuroprotective properties against glutamate toxicity through antioxidant effects, thus preventing apoptosis [7].
Moreover, since both 25-hydroxylase and 1α-hydroxylase are present in the central nervous system, it is plausible that local production of active vitamin D (1,25-dihydroxyvitamin D) is important for normal cognitive function [8, 9]. The aim of the present study was to examine the association between 25-hydroxyvitamin D (25(OH)D) and cognitive functions in a group of Italian elderly patients with AD.
Methods
This was a retrospective study, performed on 150 patients, attending our Geriatric Outpatient Clinics for cognitive disorders with diagnosis of AD. The inclusion criteria were: age 65 years old or older. Cognitive functions were assessed by MMSE (Mini Mental State Examination), functional dependence by scores on the ADL (Activities of Daily Living) and the IADL (Instrumental Activities of Daily Living). Blood samples were taken in a fasting state 25 (OH) D may fluctuate seasonally, it was only determined in blood samples collected between January and Juen. Information on education level, smoking status, precence of chronic disease was collected using questionnaires.
Statistical Analyses
Population characteristics are reported as mean ± standard deviation (SD) or percentages. All analyses were adjusted for age, sex, BMI, education, smoking and alcohol consumption. All analyses were performed using the Statistical Package for the Social Sciences software program version 18.0 for Windows (SPSS Inc, Chicago, IL).
Results
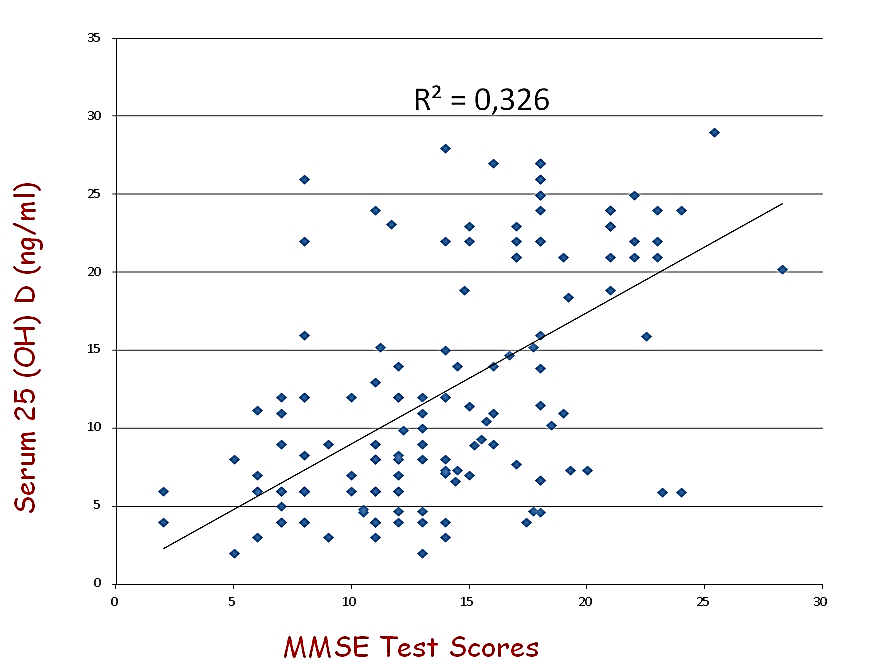
General characteristics of the study population are presented in (Table 1). The mean ± SD 25 (OH)D level of the total population was 12,27 ±7,46 ng/ml, in our sample hypovitaminosis D was present in 100% of the screened patients and ranges below 20 mg/dl were observed in 74% of the partecipant. Mean MMSE of the population was 13,83±5,18. Our data, demonstrate a positive relationship between circulating 25 (OH)D concentration and the MMSE test scores in AD (r=0,571, p=0,000, Figure 1). After dividing people according to 25(OH)D serum levels, (≥ or < 20 ng/ml), MMSE appeared significantly higher in subjects with 25(OH)D serum levels ≥ 20 ng/ml than in those inferior to 20 ng/ml (18,42+4,33 vs 12,22+4,44; p=0,000, Figure 2). After bivariate analysis, we created multiple linear regression models, including the selected variables, in order to assess MMSE change predictors (vitamin D, education, systolic blood pressure, mean blood pressure and pulse pressure).
Table 1: Characteristics of the sample studied.
|
No. |
150 |
|
No. with 25 (OH) D< 20ng/ml, |
111 (74%) |
|
Age, years |
78,71+6,05 |
|
Sex, M % |
24% |
|
Education ≤5 years, % |
84,5% |
|
MMSE |
13,8+5,18 |
|
ADL |
3,96+1,22 |
|
IADL |
2,61+1,69 |
|
Smoking,% |
5% |
|
Cardiovacular Diseases, % |
20,1% |
|
25 (OH) D ng/ml |
12,26+7,64 |
Figures 1: Relationship between MMSE Test scores and circulationg 25 (OH) D serum levels.
Figures 2: MMSE Test scores, after dividing people according to 25(OH) D serum levels.
In the model studied, vitamin D was the main predictor for MMSE changes (β=0.455, p<0.000). Therefore, the positive, significant correlation between 25 (OH) D concentration and MMSE score suggests the possibility that vitamin D may play a specific role in cognitive function in older adults. Measurement of circulating 25 (OH)D concentration is recognized as the best functional measure of vitamin D status [10]. Though the correct definition of vitamin D deficiency remains at present controversial, it is increasingly accepted that a serum 25 (OH)D concentration below 30 ng/ml is suboptimal [11]. Using this definition, all patient in this study had vitamin D deficiency. However, low vitamin D status has previously been reported in patient with AD [12, 13].
Discussion
A beneficial effect of vitamin D for cognition potentially could be mediated through a number of mecchanisms. It has been proposed that vitamin D may reduce the accumulation of Aβ42 peptide with an increase in the number of macrophages and polymorphonuclear leukocytes by VDR-dependent immunoregulation, specifically the phagocytosis and clearance of amyloid β peptide by macrophages [14-16]. Vitamin D-enhanced calcium homeostasis also could protect against neurodegeneration. Down-regulation of L-type voltage-sensitive Ca2+ channels in hippocampal neurons has been osserved in the presence of 1,25(OH)2 D3 correlating with the neuroprotective effect against excitotoxic insults [7]. Induction of neuroprotective calcium binding proteins could promote calcium homeostasis in the brain. This has been observed with the increase in parvalbumin in rat caudate putamen in response to vitamin D treatment [17]. Another vitamin D associated cytosolic protein, calbindin- D (28k), has also been found to regulate intra-cellular calcium concentration in neurons, and shows reduced levels in the hippocampal tissue in Alzheimer’s patient [18].
Additionally, vitamin D plays a role in the cerebral processes of detoxification by interacting with reactive oxygen and nitrogen species, especially in case of excessive entry of calcium into brain neurons [5]. Calcium not stored in the endoplasmic reticulum causes the activation of nitric oxide (NO) synthase and the synthesis of NO or the stimulation of phospholipase A2, the generation of superoxide anion (O2-) [19, 20]. NO can interact with O2- to form peroxynitrite (OONO –) . Oxy-reduction reactions resulting from free radicals induce dose- dependent neuronal damage to deoxyribonucleic acid, membrane lipid by peroxidation, and enzyme inactivation. The consequences are cell contraction, relocation of organules, condensation of chromatin, nuclear fragmentation, and production of apoptotic bodies containing fragments of cytoplasm and kernel, that defines neuronal apoptosis [19, 20]. The action of detoxification of vitamin D was described on cultured rat mesencephalic cells, with an efficient protection against the superoxide ion, hydrogen peroxide, and intracellular free radicals generated by reactive oxygen species (ROS) [21].
In addition, it has been demonstrated that vitamin D inhibits the synthesis of inducible nitric oxide synthase (NOS), an enzyme produced in the Central Nervous System (CNS) cells in response to stress, the high-dose action of which results in neuronal cell alteration [14]. The consequence of vitamin D administration is an increase in the number of survival neurons after exposure to cytotoxic stimuli. Another relatively direct effect could be through increased neurotrophin synthesis (NGP), as suggest at finding that 1,25(OH)D stimulate synthesis of nerve growth factor glial cell line derived neurotropic factor (GDNF) and neurotrophin (NT3) in varius non clinical studies [22-26]. Vitamin D-related trophic induction seems to play a neuroprotective role in cerebral ischemia as well as an antineurodegenerative role for dopaminergic cells in experimental animal models of Parkinson’s disease [14, 22, 27].
Finally, a more direct effect might derive through the increase in acetylcholine concentrations in the brain (CAT), as suggested by the finding that 1,25 (OH)2 D3 treatment incrementes choline acetyltransferase activity in specific rat brain nuclei [28]. CAT Keys a remorkable role in acetilcholine synthesis (Ach).
Conclusion
Our results showed a relationship between 25(OH)D and cognitive impairment in patients with AD, suggesting that 25(OH)D could be involved in the onset of dementia. Clearly, an association between low vitamin D status and cognitive impairment does not establish that vitamin D deficiency causes cognitive impairment. Additional investigatation of this clinical observation, particularly with intervention al studies, is closely requested.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 02, May 2020Accepted: Sat 16, May 2020
Published: Sat 23, May 2020
Copyright
© 2023 Alberto Castagna. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.02.07
Author Info
Alberto Castagna Carmen Ruberto Giovanni Ruotolo Giuseppe Attisani Laura Greco Raffaele Costa Rosa Paola Cerra
Corresponding Author
Alberto CastagnaCenter for Cognitive Disorders and Dementia, Azienda Sanitaria Provinciale di Catanzaro, Catanzaro, Italy
Figures & Tables
Table 1: Characteristics of the sample studied.
|
No. |
150 |
|
No. with 25 (OH) D< 20ng/ml, |
111 (74%) |
|
Age, years |
78,71+6,05 |
|
Sex, M % |
24% |
|
Education ≤5 years, % |
84,5% |
|
MMSE |
13,8+5,18 |
|
ADL |
3,96+1,22 |
|
IADL |
2,61+1,69 |
|
Smoking,% |
5% |
|
Cardiovacular Diseases, % |
20,1% |
|
25 (OH) D ng/ml |
12,26+7,64 |
References
- Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362: 329-344. [Crossref]
- Clodomiro A, Gareri P, Puccio G, Frangipane F, Lacava R et al. (2013) Somatic comorbidities and Alzheimer's disease treatment. Neurol Sci 34: 1581-1589. [Crossref]
- Castagna A, Attisani G, Gareri P, Lacava R, Cotroneo AM et al. (2014) Relation of Serum 25 (OH) to pulse pressure in Alzheimer Disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association P568-P570.
- Annweiler C, Allali G, Allain P, Bridenbaugh S, Schott AM et al. (2009) Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol 16: 1083-1089. [Crossref]
- Annweiler C, Schott AM, Berrut G, Chauvire V, Le Gall D et al. (2010) Vitamin D and ageing: neurological issues. Neuropsychobiology 62: 139-150. [Crossref]
- Manzo C, Castagna A, Palummeri E, Traini E, Cotroneo AM et al. (2016) Relationship between 25-hydroxy vitamin D and cognitive status in older adults: the COGNIDAGE study. Recenti Prog Med 107: 75-83. [Crossref]
- Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW et al. (2001) Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 21: 98-108. [Crossref]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ et al. (2001) Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. Clin Endocrinol Metab 86: 888-894. [Crossref]
- Hosseinpour F, Wikvall K (2000) Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem 275: 34650-34655. [Crossref]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (1997) Dietary Reference Intakes for calcium phosphorus, magnesium, vitamin D and Fuoride. Washington (DC): National Academies Press (US). [Crossref]
- Dawson Hughes D, Heaney RP, Holick MF, Lips P, Meunier PJ et al. (2005) Estimates of optimal vitamin D status. Osteoporos Int 16: 713-716. [Crossref]
- Sato Y, Iwamoto J, Kanoko T, Satoh K (2005) Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer's disease: a randomized controlled trial. J Bone Miner Res 20: 1327-1333. [Crossref]
- Sato Y, Asoh T, Oizumi K (1998) Retraction notice to "High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer's disease. Bone 23: 555-557. [Crossref]
- Garcion E, Wion Barbot N, Montero Menei CN, Berger F, Wion D (2002) New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 13: 100-105. [Crossref]
- Moore ME, Piazza A, McCartney Y, Lynch MA (2005) Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans 33: 573-577. [Crossref]
- Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J et al. (2009) 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 17: 703-717. [Crossref]
- De Viragh PA, Haglit KG, Celio MR (1989) Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc Natl Acad Sci U S A 86: 3887-3890. [Crossref]
- Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR et al. (1992) Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res 13: 239-250. [Crossref]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S et al. (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961-973. [Crossref]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl- D- aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A 92: 7162-7166. [Crossref]
- Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H et al. (2001) Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 40: 761-771. [Crossref]
- Cornet A, Baudet C, Neveu I, Baron Van Evercooren A, Brachet P et al. (1998) 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res 53: 742-746. [Crossref]
- Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D (1994) 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 24: 70-76. [Crossref]
- Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R et al. (1991) 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 28: 110-114. [Crossref]
- Naveilhan P, Neveu I, Wion D, Brachet P (1996) 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 7: 2171-2175. [Crossref]
- Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M (1994) 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 6: 124-126. [Crossref]
- Wang Y, Chiang YH, Su TP, Hayashi T, Morales M et al. (2000) Vitamin D3 attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology 39: 873-880. [Crossref]
- Sonnenberg J, Luine VN, Krey LC, Christakos S (1986) 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology 118: 1433-1439. [Crossref]

