Journals
The Effects of Vitamin D3, Vitamin B12, and Vitamin K on the Differentiation of Porcine Adipose-Derived Stem Cells (ASC) into Osteoblasts
A B S T R A C T
Large bone defects present a tremendous challenge to the treating surgeon. Tissue engineering using scaffolds of various sizes and shapes that contain stem cells and other osteoinductive molecules offer a potential solution to this difficult problem. The aim of this project was to evaluate if standard osteogenic medium infused with either vitamin D3, vitamin K, or vitamin B12 influences the differentiation of adipose-derived stem cells (ASC) into osteoblasts. Only the 100 nM vitamin D3 treatment had a positive influence on ASC differentiation into osteoblasts. Moreover, we found higher levels (statistically significant) of osteocalcin production in all vitamin groups compared with the standard osteogenic medium.
Keywords
ASC, differentiation, osteogenesis, osteocalcin, vitamins, regeneration
Introduction
The process of osteogenesis occurs through endochondral or intramembranous ossification. During the former, mesenchymal cells differentiate to form a cartilage matrix that will progressively become more bone-like after other stem cells invade the matrix and undergo osteogenesis. In contrast, intramembranous ossification involves mesenchymal stem cells (MSCs) directly differentiating into osteoblasts in situ [1]. Large bone defects from traumatic injury or tumor resection present a challenge to both the treating surgeon and to the healthcare system. About 10% of those injuries will require surgical intervention because of non-union, and these injuries will cost somewhere around $2.5 billion per year [2, 3]. Autologous bone graft has been the gold standard therapeutic method for many years, but the surgeon is limited by size and shape. Structural allografts provide initial stability but are very slow to incorporate by creeping substitution. These allografts sometimes fail to completely integrate and will eventually suffer fatigue failure. Metal implants such as rods or plates and screws would also suffer from eventual fatigue failure [4]. Factoring inpatient complications and revision surgeries to correct for fatigue failure, the total cost for the injuries is much higher than the initial medical treatment. Tissue engineering with a biological scaffold offers a viable solution to these problems since the scaffold can come in a variety of shapes and sizes. In addition, different cells can be injected into the scaffold to promote osteogenesis – primarily mesenchymal stem cells (MSC) that can differentiate into osteoblasts and other types of progenitor cells.
MSCs can be isolated from either bone marrow (BMSC) or adipose tissue (ASC). There is a myriad of reasons why ASCs are more viable for tissue engineering therapies when compared to BMSCs; however, both are capable of differentiating towards osteogenic, myogenic, chondrogenic, and adipogenic lineages [5]. ASCs are preferable to BMSCs because harvesting adipose tissue for ASCs is safer and results in more stem cells than harvesting cells from other tissues such as muscle or bone marrow. Additionally, ASCs are more proliferative. As an example, flasks seeded with about 3,500 ASCs will reach confluency in the same amount of time as a flask seeded with anywhere between 20,000 to 40,000 BMSCs. The highly proliferative nature of ASCs makes them ideal for both in vitro and in vivo experiments [6]. Finally, past work as shown that ASCs have robust mineralization within one week of osteogenic differentiation done in vitro [4].
To stimulate osteoblast formation from ASCs, osteoinductive factors have been used in conjunction with an osteoconductive matrix. The bone that develops from differentiated ASCs can grow through a structural matrix that is made of collagen. This process represents the osteoconductive aspect of tissue engineering therapy. Osteoinductive factors include various vitamins and minerals that aid in stem cell differentiation independent of a scaffold [7]. The literature indicates that certain vitamins, such as D3, B12, and K have osteoinductive effects. Vitamin D3 (cholecalciferol) is a fat-soluble secosteroid that increases intestinal absorption of calcium, iron, magnesium, phosphate, and zinc. Vitamin D3 deficiencies can lead to overt rickets in children, osteoporosis from increased calcium reabsorption, and muscle weakness for adults with osteomalacia [8]. In typical bone formation, osteoblasts secrete a collagen matrix that provides structural support in which the bone can mature. Without cholecalciferol, the collagen matrix has a mineralization defect. This means that the matrix does not provide the necessary structural support for osteogenesis [9, 10]. Vitamin B12 (cobalamin) is a water-soluble vitamin that acts as a coenzyme in energy-producing metabolic pathways, in addition to preserving nervous system functions [11].
The literature remains unclear as to whether cobalamin provides either a positive effect on osteoblast proliferation or has no effect [12]. It has been shown that B12 deficiency stimulates osteoclastogenesis in vitro through an indirect pathway involving increased levels of methylmalonic acid and homocysteine [13]. Vitamin K is a fat-soluble vitamin required for completing the synthesis of certain proteins necessary for osteogenesis, such as osteocalcin. Once vitamin K modifies those proteins, they are capable of binding calcium ions. Vitamin K has been shown to decrease fracture rates and osteoporosis incidence, thus improving bone health [14]. In addition, vitamin K and vitamin D3 have been shown to work synergistically to improve bone density [15, 16]. Large animal models, such as swine, traditionally have been used in the development or improvement of therapeutic protocols. Swine models have similar structure and physiology in comparison to humans, and they are a plentiful source of MSCs. Thus, the osteoblast formation from pig derived ASCs under certain conditions can be used as an indication of human ASC behavior under similar conditions [17, 18].
When grown in culture with osteogenic differentiation media, ASCs differentiate into osteoblasts that are characterized by calcium deposits and bone matrix markers [19]. The aim of our study was to determine if exposure to increased levels of vitamin D3, vitamin B12, or vitamin K can affect the formation of osteogenic nodules in ASC cultures, as measured using Alizarin red staining and pig osteocalcin ELISA assay.
Materials and Methods
I Experimental Design
To study vitamin D3, adipose-derived stem cells were plated at a concentration of 50,000 cells/mL, and then subjected to 5 treatments of vitamin D3; 1000 nM, 500 nM, 100 nM, 50 nM, 10 nM; a positive standard osteogenic medium control; and a negative DMEM control. The experiment lasted for four weeks, with media being changed twice a week to maintain cell nourishment and development. Finally, the cells were stained with alizarin red. These stains allowed us to determine whether any concentration of vitamin D3 affected the differentiation of osteoblasts. A total of 9 distinct replicates (cell line from three pigs were used and grown in triplicate) were grown and stained. This same procedure was repeated for vitamin K and vitamin B12. Adipose-derived stem cells in the vitamin K experiment were subjected to 5 treatments of vitamin K: 1000 nM, 500 nM, 100 nM, 50 nM, and 10 nM. Adipose derived stem cells in the vitamin B12 experiment were subjected to 6 treatments of vitamin B12: 20 µM, 10 µM, 2 µM, 1 µM, 0.2 µM, and 0.1 µM.
The second part of the experiment was aimed at determining the quality of the osteoblasts produced in the most promising treatments from each vitamin. The spent medium was saved after the fourth week of the experiment and analyzed with a pig osteocalcin ELISA kit. Using this kit, we were able to determine the osteocalcin concentration in the experimental medium compared to the concentration in the standard osteogenic medium, which would determine the quality of the osteoblasts. In total, 5 samples from each vitamin were run in triplicate on the ELISA kit to determine osteocalcin concentrations.
II ADSC Isolation and Culture in Vitro
Subcutaneous back fat and bone marrow were acquired from 3 castrated Yorkshire crossbred male pigs, at approximately 6 months of age, under protocols approved by the University of Illinois Institutional Animal Care and Use Committee (IACUC). Pigs were euthanized at the University of Illinois Meat Science Laboratory abattoir. The skin overlying the loin area was shaved to remove the hair and scrubbed three times using Betadine® solution (Povidoneiodine, 10% - Purdue Products L.P., Cranbury, NJ), and three times with 70% ethanol (Aaper Alcohol and Chemical Co. Shelbyville, KY) to avoid contamination of the sample. A square of subcutaneous back fat (~ 100 cm2) was then excised from the sanitized area from each pig, placed in a sterile plastic bag, transported to the laboratory on ice and placed at 4ºC until cell harvest (< 1 hr). The subcutaneous fat was dissected from the skin with a sterile scalpel. All the surfaces of the fat, which were originally exposed at tissue harvest, were also trimmed off with a sterile scalpel blade so that only sterile fat was processed for cell isolation. Strips of sterile fat were washed twice in Dulbecco’s Phosphate Buffer Saline (DPBS, Sigma Aldrich D5773, St. Louis, MO) containing 1% Penicillin G-Streptomycin (Sigma P3539) and 5.0 mg/L of Amphotericin B (Sigma A9528). After washing, tissue was minced with scalpel blades and then digested with 0.075% collagenase type I-A (Sigma C2674) in DPBS, in a 50 mL conical tube (Corning, NY) (v/v - tissue/collagenase), in the incubator at 37°C for 90 min. The conical tubes, containing fat and collagenase, were vigorously shaken every 10 to 15 min to ensure uniform digestion. After digestion, tubes were centrifuged at 200 x g for 10 min at room temperature. The buoyant cell fraction and supernatant were discarded, and 2 mL of red blood cell lysis buffer (Sigma R7757) was added to the pellet and gently mixed for 2 min. Subsequently, 20 mL of DPBS was added to the tubes and were centrifuged at 200 x g for 5 min at room temperature, to obtain a cell pellet that was then resuspended in the culture medium.
The culture medium used was high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma D5648), supplemented with 10% Fetal Bovine Serum (FBS, BenchMark™, Gemini Bio –Products, West Sacramento, CA), plus 1% Penicillin G-Streptomycin and 5.0 mg/L of Amphotericin B. Cells were counted using a hemocytometer, plated in 75 cm2 Corning cell culture flasks at 7.5 x 105 cells in 15 mL of culture medium, and incubated at 39°C and 5% CO2 in 100% humidified air. The medium was changed every other day until the initial cell culture passage. Passage 0 cells reached confluence at approximately day 10 of culture. In order to keep the cells at a sufficiently low density to stimulate further growth, the initial cell cultures were washed using DPBS and harvested by digestion with 0.25% Trypsin (Sigma T4799) - 0.04% EDTA (Sigma E6753) for 3 min. Trypsin was then inactivated by adding an equivalent volume of culture medium, and the cells were centrifuged at 200 x g for 5 min at room temperature. Cells were resuspended in culture medium for plating in 75 cm2 cell culture flasks at a density of approximately 7.5 x 105 cells/75 cm2. These passage 1 cells were 80% confluent after 4 days. Cells were trypsinized, as described above, and frozen at 3 x 106 cells per 1.2 mL in cryogenic vials (Nalgene® Labware, Rochester, NY). Freezing medium consisted of 75% DMEM supplemented with 15% FBS and 10% dimethyl sulfoxide (DMSO, Sigma D2650). Vials were placed in Nalgene Cryo 1ºC Freezing containers (Nalgene® Labware) and placed in a -80ºC freezer. On the following day, cells were transferred to liquid nitrogen and stored until further use.
The base of the osteogenic medium used was made with 100 mL of high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma D5648), 100 µL of Ascorbic acid-2-phosphate (AA2P)(Sigma 43752) at a concentration of 50 µg/mL, 1 mL of Beta Glycerophospate (10mM), and 10µL of Dexamethasone (100 nM). Once the media was complete, zinc sulfate heptahydrate (ZnSO4. 7H2O) was added to give 6 concentrations: 8mM, 4mM, 0.8mM, 0.4mM, 0.08mM, and 0.04mM. These 6 treatments, along with a negative DMEM control and a positive standard osteogenic control, were plated at a concentration of 5*105 cells/mL of respective medium and changed twice a week for a four-week period.
III Alizarin Red S Staining
Alizarin Red S staining is a widely used technique that evaluates the presence of calcium-rich deposits formed by cells in culture. It is especially useful because the dye can be extracted from the stained monolayer and assayed [20]. At the end of the four-week period, cells were washed with Dulbecco’s Phosphate Buffer Saline (DPBS, Sigma Aldrich D5773, St. Louis, MO) containing 1% Penicillin G-Streptomycin (Sigma P3539) and 5.0 mg/L of Amphotericin B (Sigma A9528), then exposed to 10% buffered formalin for 30 minutes to fix the cells. Once fixed, cells were washed with Nanopure autoclaved H2O (dd H2O), and then stained with a 2% Alizarin Red S stain, pH 4.5, to stain calcium producers. After 45 minutes, Alizarin Red S stain was removed, and stains were washed four times with ddH2O, then stored in DPBS. Nodules that were stained red were counted and evaluated on an Olympus inverted cell culture microscope, and images were acquired using a Zeiss camera and running Zen Software (Zeiss, Germany). Data were analyzed by analysis of variance using the Generalized Linear Model (GLM) procedure (SPSS 24 - IBM). Bonferroni's post hoc test was used to perform multiple statistical comparisons. The alpha level was set at 0.05.
IV Porcine Osteocalcin ELISA
ELISA for porcine osteocalcin (Catalog No. LS-F5374) was performed using a porcine osteocalcin sandwich ELISA Kit (LifeSpan BioSciences Inc, LS-F5374) according to the protocol provided by LifeSpan BioSciences, Inc. A Lyophilized Standard was diluted in a one-half manner and added to the first two lanes of the supplied 96-well Strip plate, pre-coated with target specific capture antibodies. Thawed cell culture supernatants were centrifuged and added into the plate, then incubated at 37ºC for 1 hour. Samples were aspirated off, then followed by a biotinylated detection antibody (diluted at a ratio of 1:100), followed by hour incubation at 37ºC. This antibody was aspirated, and the plate was washed with a 1X wash buffer 3 times before an Avidin-Horseradish Peroxidase (diluted at a ratio of 1:100) was added to the wells and incubated for 30 minutes at 37ºC. This was aspirated, and the plate was washed 5 times with the 1X buffer, after which a TMB substrate was added to react with the HRP enzyme and bring color development. Once color development reached its peak point, sulfuric acid was added to stop color development, and the plate was analyzed under a plate reader set to 450nm. All recorded parameters were subjected to a Student’s t-test. The alpha level was set at 0.05.
Results
I In Vitro Alizarin Red S and von Kossa Stain
Using methods as dictated by Monaco and colleagues we were able to separate nodules into one of two groups: formed and forming nodules [19, 21, 22]. The results for Vitamin D3 indicate that only the 100 nM treatment had a positive effect on differentiation (Table 1). The other vitamin D3 treatments formed nodules, but there was no statistically significant difference in the number of nodules compared to the standard osteogenic control. When we evaluated the vitamin B12 treatments, we did not find any statistically significant difference between the control and the experimental groups. Furthermore, the 20 µM treatment yielded a significantly lower number of nodules than the standard osteogenic control (Table 2). Similarly, vitamin K, in all its concentrations, did not show statistically significant difference; none of the vitamin K treatments had a positive effect on differentiation. Additionally, the 1000 nM K treatment yielded significantly less nodules than the standard osteogenic control (Table 3).
Table 1: Number of formed and forming nodules observed per vitamin D3 treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
1000 nM |
2.2 (1.5)A |
1.7 (0.5)A |
3.8 (1.3)A |
|
500 nM |
3.3 (3.4)A |
2.2 (1.6)A |
5.5 (4.8)A |
|
100 nM |
5.2 (2.6)B |
2.5 (2.3)A |
7.7 (4.2)B |
|
50 nM |
2.3 (1.2)A |
2.5 (1.6)A |
4.8 (1.7)A |
|
10 nM |
1.3 (1.5)A |
1.3 (1.8)A |
2.7 (3.1)A |
|
DMEM |
0C |
0.3 (0.5)C |
0.3 (0.5)C |
|
Osteogenic Media |
2.3 (2.1)A |
1.8 (1.5)A |
4.2 (2.1)A |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
Table 2: Number of formed and forming nodules observed per vitamin B12 treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
20 µM |
0A |
1.2 (1.5)AB |
1.2 (1.5)A |
|
10 µM |
2.8 (1.7)B |
2.2 (1.5)AB |
5.0 (3.0)B |
|
2 µM |
2.3 (0.8)B |
1.5 (0.8)AB |
3.8 (1.2)B |
|
1 µM |
2.8 (1.5)B |
2.0 (1.8)AB |
4.8 (2.5)B |
|
0.2 µM |
1.7 (1.6)B |
1.2 (1.0)AB |
2.8 (2.2)AB |
|
0.1 µM |
2.5 (3.2)B |
2.7 (3.0)B |
5.2 (6.1)B |
|
DMEM |
0A |
0.3 (0.5)A |
0.3 (0.5)A |
|
Osteogenic Media |
2.3 (2.1)B |
1.8 (1.5)AB |
4.2 (2.1)B |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
Table 3: Number of formed and forming nodules observed per vitamin K treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
1000 nM |
0.7 (1.6)bc |
0.2 (0.4)aA |
0.8 (2.0)A |
|
500 nM |
3.2 (1.2)B |
1.7 (1.6)bB |
4.8 (1.6)aB |
|
100 nM |
3.0 (1.9)B |
1.5 (1.2)bB |
4.5 (2.9)aB |
|
50 nM |
2.5 (2.8)aBC |
2.0 (2.3)bB |
4.5 (4.8)aB |
|
10 nM |
1.5 (2.7)AB |
0.8 (1.2)aA |
2.3 (2.5)bB |
|
DMEM |
0A |
0.3 (0.5)aA |
0.3 (0.5)aA |
|
Osteogenic Media |
2.3 (2.1)aB |
1.8 (1.5)B |
4.2 (2.1)B |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.01).
abcLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
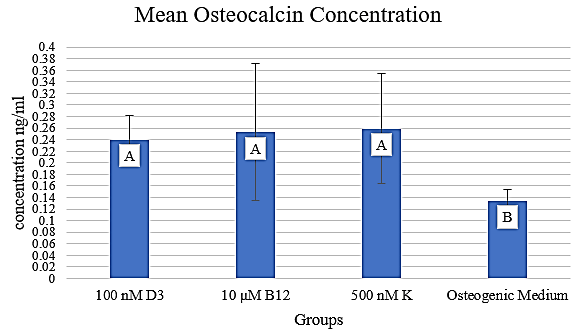
Figure 1: Mean osteocalcin concentration in media containing 100 nM D3, 10 µM B12, and 500 nM K, respectively. The concentration is expressed in ng/ml.
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.01).
II ELISA Measurement of Osteocalcin Expression
Osteocalcin levels were assayed from samples corresponding to the most promising concentrations from each vitamin: 100 nM D3, 10 µM B12, and 500 nM K (Figure 1). Least Significant Difference (LSD) analysis demonstrated that cultures incubated in each vitamin produced significantly more osteocalcin compared to those in osteogenic media alone (p <.01). However, the cultures incubated in the vitamin treatments did not produce significantly different amounts of osteocalcin when compared to each other (p >.01). These findings suggest that there is a difference in the quality of nodules formed in the experimental groups compared to those in the osteogenic medium without any added vitamins.
Discussion
Changing a medium’s chemical composition can alter cellular activity. This change can involve either altering levels of preexisting media components or adding a new component. At the same time, the effect of micronutrients like vitamins is not well known [23]. This study focuses on the latter in the hopes that adding a new component will enhance the osteogenic differentiation of ASCs. The first step of this study was to find the therapeutically relevant range of concentrations for vitamin D3, vitamin K, and vitamin B12. The second step was to evaluate the effect of vitamin supplementation on osteogenic differentiation of ASC. The importance of all three vitamins for human physiology is well established in the literature. Specifically, vitamin D3 enhances intestinal absorption of calcium, phosphate, and other minerals that will aid in bone development vitamin K is required to synthesize osteocalcin, an important protein necessary for proper bone health and vitamin B12 deficiencies lead to increased osteoclastogenesis and therefore poor bone density [9, 10, 13, 14].
In this study, we showed the positive influence of vitamin D3 supplementation on stem cell differentiation: there was an increase in the formation of osteogenic nodules in differentiating ASC cultures under the 100 nM D3 treatment (7.7 vs. 4.2 D3 and standard osteogenic medium, respectively). These results parallel findings where dental bud stem cells (DBSCs) were cultured with 10 nM 1α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) [24]. Posa and coworkers demonstrated the positive effect of supplementing the osteogenic medium with vitamin D3 on osteogenesis of DBSC cultures. In parallel, when we evaluated the effect of vitamin B12, we did not find a concentration that produced nodules that was significantly higher compared with the control. In the literature, there are few reports regarding the effect of vitamin B12 on stem cells. In 2006, there was one report that showed a positive effect of vitamin B12 when cultured with embryonic stem cells [25]. Our results showed the detrimental effect of vitamin B12 at concentrations above 10 µM; however, below10 µM, nodule formation was similar to the control. Finally, vitamin K is known to have an active role in bone formation and osteoblast metabolism [26]. However, our results do not show an increase in the number of nodules compared with the osteogenic base medium. Instead, a detrimental effect was noted at the concentration of 1000 nM. The results summarized show that only vitamin D3 increased the number of nodules formed. However, when we went on to evaluate the quality of the osteoblasts, we found positive effects of the vitamins in the three experimental groups showing a statistically significant increase (p<0.01) in the amount of osteocalcin produced.
Conclusion
We have demonstrated that supplementing standard ASC osteogenesis-promoting media with 100 nM vitamin D3 has a positive effect on the differentiation of porcine ASCs into osteoblasts We have also shown that supplementation with 1000 nM vitamin K has a negative effect on porcine ASC during the differentiation into osteoblasts, whereas the other concentrations had no effect. Similarly, supplementation with 20 µM vitamin B12 has a negative effect on porcine ASC differentiation into osteoblasts, whereas the other concentrations had no effect. The concentration of osteocalcin in the 10 µM vitamin B12 and 500 nM vitamin K groups, respectively, was larger relative to that in the osteogenic medium.
Acknowledgements
The authors would like to thank Molly Sermersheim for carefully reading of the manuscript. This work was partially supported by the USDA Multistate Project W-4171 (MBW), the University of Illinois Agricultural Experiment Station and the Ross Foundation (MBW).
Article Info
Article Type
Research ArticlePublication history
Received: Mon 20, Apr 2020Accepted: Tue 05, May 2020
Published: Mon 11, May 2020
Copyright
© 2023 Matthew B. Wheeler. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JBEM.2020.01.01
Author Info
Bane T.A. Bertels J.C. Matthew B. Wheeler Polkoff K.M. Rubessa M.
Corresponding Author
Matthew B. WheelerDepartment of Animal Sciences, University of Illinois, Urbana-Champaign, Urbana, Illinois, USA
Figures & Tables
Table 1: Number of formed and forming nodules observed per vitamin D3 treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
1000 nM |
2.2 (1.5)A |
1.7 (0.5)A |
3.8 (1.3)A |
|
500 nM |
3.3 (3.4)A |
2.2 (1.6)A |
5.5 (4.8)A |
|
100 nM |
5.2 (2.6)B |
2.5 (2.3)A |
7.7 (4.2)B |
|
50 nM |
2.3 (1.2)A |
2.5 (1.6)A |
4.8 (1.7)A |
|
10 nM |
1.3 (1.5)A |
1.3 (1.8)A |
2.7 (3.1)A |
|
DMEM |
0C |
0.3 (0.5)C |
0.3 (0.5)C |
|
Osteogenic Media |
2.3 (2.1)A |
1.8 (1.5)A |
4.2 (2.1)A |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
Table 2: Number of formed and forming nodules observed per vitamin B12 treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
20 µM |
0A |
1.2 (1.5)AB |
1.2 (1.5)A |
|
10 µM |
2.8 (1.7)B |
2.2 (1.5)AB |
5.0 (3.0)B |
|
2 µM |
2.3 (0.8)B |
1.5 (0.8)AB |
3.8 (1.2)B |
|
1 µM |
2.8 (1.5)B |
2.0 (1.8)AB |
4.8 (2.5)B |
|
0.2 µM |
1.7 (1.6)B |
1.2 (1.0)AB |
2.8 (2.2)AB |
|
0.1 µM |
2.5 (3.2)B |
2.7 (3.0)B |
5.2 (6.1)B |
|
DMEM |
0A |
0.3 (0.5)A |
0.3 (0.5)A |
|
Osteogenic Media |
2.3 (2.1)B |
1.8 (1.5)AB |
4.2 (2.1)B |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
Table 3: Number of formed and forming nodules observed per vitamin K treatment.
|
Treatment |
Formed Nodule |
Forming Nodule |
Total Nodules |
|
1000 nM |
0.7 (1.6)bc |
0.2 (0.4)aA |
0.8 (2.0)A |
|
500 nM |
3.2 (1.2)B |
1.7 (1.6)bB |
4.8 (1.6)aB |
|
100 nM |
3.0 (1.9)B |
1.5 (1.2)bB |
4.5 (2.9)aB |
|
50 nM |
2.5 (2.8)aBC |
2.0 (2.3)bB |
4.5 (4.8)aB |
|
10 nM |
1.5 (2.7)AB |
0.8 (1.2)aA |
2.3 (2.5)bB |
|
DMEM |
0A |
0.3 (0.5)aA |
0.3 (0.5)aA |
|
Osteogenic Media |
2.3 (2.1)aB |
1.8 (1.5)B |
4.2 (2.1)B |
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.01).
abcLeast square means (±SD) within each column without common superscripts differ significantly (p<0.05).
ABLeast square means (±SD) within each column without common superscripts differ significantly (p<0.01).
References
- Shahi M, Peymani A, Sahmani M (2017) Regulation of Bone Metabolism. Rep Biochem Mol Biol 5: 73-82. [Crossref]
- Laurencin C, Khan Y, El Amin SF (2006) Bone graft substitutes. Expert Rev Med Devices 3: 49-57. [Crossref]
- Wu N, Lee Y, Segina D, Murray H, Wilcox T et al. (2013) Economic burden of illness among US patients experiencing fracture nonunion. Orthop Res Rev 5: 21-33.
- Levi B, James AW, Nelson ER, Vistnes D, Wu B et al. (2010) Human adipose derived stromal cells heal critical size mouse calvarial defects. PloS One 5: e11177. [Crossref]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279-4295. [Crossref]
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24: 150-154. [Crossref]
- Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10 Suppl 2: S96-101. [Crossref]
- Holick MF (2003) Vitamin D deficiency: what a pain it is. Mayo Clin Proc 78: 1457-1459. [Crossref]
- Holick MF (2009) Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol 19: 73-78. [Crossref]
- Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S et al. (2000) Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med 247: 260-268. [Crossref]
- McLean RR, Hannan MT (2007) B vitamins, homocysteine, and bone disease: epidemiology and pathophysiology. Curr Osteoporos Rep 5: 112-119. [Crossref]
- Dai Z, Koh WP (2015) B-vitamins and bone health--a review of the current evidence. Nutrients 7: 3322-3346. [Crossref]
- Vaes BL, Lute C, Blom HJ, Bravenboer N, de Vries TJ et al. (2009) Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif Tissue Int 84: 413-422. [Crossref]
- Yonemura K, Kimura M, Miyaji T, Hishida A (2000) Short-term effect of vitamin K administration on prednisolone-induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcif Tissue Int 66: 123-128. [Crossref]
- Iwamoto J, Takeda T, Ichimura S (2000) Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci 5: 546-551. [Crossref]
- Somekawa Y, Chigughi M, Harada M, Ishibashi T (1999) Use of vitamin K2 (menatetrenone) and 1,25-dihydroxyvitamin D3 in the prevention of bone loss induced by leuprolide. J Clin Endocrinol Metab 84: 2700-2704. [Crossref]
- Kozlik Feldmann R, Lang N, Aumann R, Lehner A, Rassoulian D et al. (2008) Patch Closure of Muscular Ventricular Septal Defects With a New Hybrid Therapy in a Pig Model. J Am Coll Cardiol 51: 1597-1603. [Crossref]
- Rubessa M, Polkoff K, Bionaz M, Monaco E, Milner DJ et al. (2017) Use of Pig as a Model for Mesenchymal Stem Cell Therapies for Bone Regeneration. Anim Biotechnol 28: 275-287. [Crossref]
- Monaco E, Bionaz M, Rodriguez Zas S, Hurley WL, Wheeler MB (2012) Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PloS One 7: e32481. [Crossref]
- Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329: 77-84. [Crossref]
- Kim D, Monaco E, Maki A, de Lima AS, Kong HJ et al. (2010) Morphologic and transcriptomic comparison of adipose- and bone-marrow-derived porcine stem cells cultured in alginate hydrogels. Cell Tissue Res 341: 359-370. [Crossref]
- Monaco E, Sobreira de Lima A, Bionaz M, Maki A, Wilson SM et al. (2009) Morphological and Transcriptomic Comparison of Adipose and Bone Marrow Derived Porcine Stem Cells. Open Tissue Eng Regen Med J 2: 20-22.
- Valle YL, Almalki SG, Agrawal DK (2016) Vitamin D machinery and metabolism in porcine adipose-derived mesenchymal stem cells. Stem Cell Res Ther 7: 118. [Crossref]
- Posa F, Di Benedetto A, Colaianni G, Cavalcanti Adam EA, Brunetti G et al. (2016) Vitamin D Effects on Osteoblastic Differentiation of Mesenchymal Stem Cells from Dental Tissues. Stem Cells Int 2016: 9150819. [Crossref]
- Yamazoe H, Kobori M, Murakami Y, Yano K, Satoh M et al. (2006) One-Step Induction of Neurons from Mouse Embryonic Stem Cells in Serum-Free Media Containing Vitamin B12 and Heparin. Cell Transplant 15: 135-145. [Crossref]
- Akbari S, Rasouli Ghahroudi AA (2018) Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed Res Int 2018: 4629383. [Crossref]
