Journals
The impact of HPV infection on risk of progression and overall mortality in vulvar squamous cell carcinoma: a retrospective single-center analysis
A B S T R A C T
Aims: To investigate the impact of Human Papillomavirus (HPV) infection on clinic and histopathologic characteristics, and prognostic factors of patients affected by vulvar squamous cell carcinoma (VSCC).
Methods: Fifty-six patients diagnosed with VSCC at the IRCCS Fondazione Policlinico San Matteo, Pavia, Italy, from March 2001 to February 2016, were enrolled in a retrospective analysis. HPV DNA was detected by the INNO-LiPA HPV genotyping assay, version EXTRA II, on corresponding pathological specimens. Clinic and histopathologic characteristics were compared through Fisher's exact test. Kaplan–Meier survival curves and Cox regression models were used to analyze prognostic factors.
Results: According to the Kaplan–Meier curves, no differences were found neither in Disease Free Survival (DFS) (p=0.221), nor in Overall Survival (OS) (p=0.135) between HPV-positive and HPV-negative patients. At Cox multivariate analysis, lymph node metastasis and positive surgical margins were significantly associated to higher risk of progression/relapse. This association was retained but decreased by lymph node metastasis (HR=5.92, 95% CI: 2.16 – 16.23; p < 0.001) and positive surgical margins (HR=3.10, 95% CI: 1.13 – 6.50; p=0.028) in case of HPV-positivity, that was instead related to lower risk of disease progression/relapse (HR=0.31, 95% CI: 0.13 – 0.75; p=0.009). Age ≥ 75 years, lymph node metastasis and adjuvant radiotherapy were statistically associated to higher mortality rate. A significant reducing effect on mortality was shown adjusting HPV for lymph node extracapsular spread (HR=0.38, 95% CI: 0.16-0.88; p=0.025), and FIGO stage > I (HR=0.42, 95% CI: 0.19 – 0.95; p=0.037), that, instead, related to higher risk of death for any cause (HR=4.46, 95% CI: 1.51 – 13.19; p=0.007 and HR=2.53, 95% CI: 1.12 – 5.73; p=0.026, respectively).
Conclusions: Risk of progression is reduced in HPV-positive patients with node metastasis and positive surgical margins. Overall, HPV infection has a protective effect on mortality in case of node extracapsular spread or FIGO stage >
Keywords
Vulvar squamous carcinoma, high-risk, HPV, FIGO stage, Surgical margins, Lymph node metastasis.
I N T R O D U C T I O N
Vulvar cancer represents less than 5% of all gynaecological malignancies, with an incidence of 2-3 per 100000 women per year in developed countries [1]. More than 90% of vulvar tumours are squamous cell carcinomas. Two different pathogenetic pathways have been historically described: one related to Human Papillomavirus (HPV) infection and another HPV-independent, that usually develops in women affected by chronic vulvar dermatosis [2, 3]. The carcinogenesis related to HPV-persistent infection accounts for 43-60% of vulvar squamous cell carcinomas (VSCCs) and is especially caused by high-risk (HR) HPV genotypes, in particular HPV 16, but also HPV 18, 31, 33 and 45 [4].
These two kinds of VSCC are characterized by different clinic and histologic features. Nevertheless, there are still poor and controversial data about the effect of HR HPV infection on prognosis, probably due to the low incidence of the disease and the heterogeneous distribution of patients regarding stage of disease and type of treatment [5, 6]. Several authors have already investigated prognostic factors for relapse and survival in patients affected by VSCC, that seem to be first related to nodal involvement [7, 8]. Some authors did not prove a significant difference of survival in patients with HPV-positive and HPV-negative vulvar cancers, whereas others highlighted a better survival rate in HPV-related carcinomas [9, 10]. A better prognosis has been previously reported in HPV-positive oropharyngeal and anal cancers and has been related to an historically-documented better response to chemo- and radiotherapy [11-13]. Nowadays, these evidences have not still been confirmed in HPV-related VSCCs.
Therefore, the aim of this study was to investigate clinic and histopathologic characteristics and prognostic factors of patients affected by VSCC and treated in a single Institute. In addition, we analysed the impact of HPV infection on characteristics of tumour, risk of relapse and survival rate, with a particular attention to HR HPV genotypes and multiple infections.
Patients and Methods
Population
All patients diagnosed with vulvar cancer, who underwent surgical treatment at the Department of Obstetrics and Gynaecology of the IRCCS Fondazione Policlinico San Matteo, University of Pavia, Italy, from March 2001 to February 2016, were retrieved from our file archives and enrolled in a retrospective analysis. The Institutional Review Board of our hospital approved the study and all patients signed an informed consent for the use of sensitive data for health research before surgery. Patients were included if the following criteria were met: (i) age at diagnosis of 18 years or older; (ii) histologic confirmation of VSCC; (iii) available data about follow-up longer than 3 months. Patients were excluded in case of non-squamous vulvar cancers.
Data regarding principal socio-demographic and clinic characteristic of patients were recorded. Macroscopic and histologic characteristics of tumour were recovered from surgical and pathological reports. Tumour stage was assigned according to the 2009 International Federation of Obstetrics and Gynecologists (FIGO) [14]. Primary treatment modality, adjuvant chemo- and/or radiotherapy as well as type, time and treatment of any recurrence were registered. Follow-up was routinely scheduled every 3-4 months during the first 2 years and thereafter every 6 months at the dedicated Vulvar Pathology Clinic of our Department. A dedicated database was prospectively filled in at each follow-up visit. To improve accuracy of survival data, telephonic interviews were allowed in case of lost to follow-up patients.
HPV-DNA detection and genotyping
For all enrolled patients, the corresponding pathological specimens were retrieved from the archives of the Department of Pathology of the IRCCS Fondazione Policlinico San Matteo. Original slides were reviewed to confirm the diagnosis according to 2004 WHO System criteria and to select formalin-fixed paraffin-embedded (FFPE) tissue blocks for DNA extraction and detection [15]. For DNA isolation from FFPE samples, 10 μM sections were incubated in 200 μL of lysis solution for 16/24 h at 56°C. After heat inactivation, the lysates were centrifuged to eliminate wax, extracted with phenol–chloroform and resuspended in 100 μL of water. HPV type-specific sequences were detected by the line probe, INNO-LiPA HPV genotyping assay, version EXTRA II (Innogenetics N.V., Ghent, Belgium) according to the manufacturer’s instructions, using an Auto-LiPA 48 instrument. The EXTRA II version of the assay allows the detection of 13 high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68), 6 intermediate-risk (26, 53, 66, 70, 73, 82), 9 low-risk (6, 11, 40, 42, 43, 44, 54, 61, 81), and 4 unclassified HPV types (62, 67, 83, 89). Co-amplification of human HLA-DPB1 sequences is included in the assay as control for DNA adequacy. Hybridization patterns were analyzed automatically using the Linear Reader and Analysis Software (LIRAS, Innogenetics); discrepant interpretations were resolved by consensus reading.
Statistical analysis
The results have been reported as medians, absolute frequencies and percentages. Fisher's exact test was used for comparisons between nominal variables. Age was considered either as ordinal or as nominal variable with a cut-off at 75 years. Disease-free survival (DFS) was defined as the time from treatment to local and/or regional recurrence or metastasis. Overall survival (OS) was defined as the time from the date of treatment to the date of death of disease or to the last follow-up visit. For inferential analysis, the follow-up of patients was truncated at 5 years. Deaths from other causes not related to vulvar cancer and without documented progression or relapse were censored at the date of death. Outcome data, including “no evidence of disease” (NED) versus progression/recurrence of disease and alive patient versus dead patient, were described by the Kaplan–Meier survival curves, that were compared by use of the log-rank test.
Univariate Cox models were used to analyze the factors studied as prognostic factors for DFS and OS using the risk estimation as hazard ratio (HR) and their 95% confidence interval (95% CI). Multivariate regression models (Cox proportional hazard model) were applied to adjust group comparisons to possible confounders regarding progression/recurrence and mortality. Multivariate regression models included all statistically significant variables based on univariate Cox models and HPV-positive status. Data were analyzed with the R freeware statistical software (R version 3.3.0). P-value of .05 or less was considered statistically significant.
Results
The final population under study was composed of 56 women. HPV-DNA was detected in 29 corresponding pathological specimens; therefore, VSCC were divided in HPV-negative (27, 48.2%) and HPV-positive (29; 51.8%). HR HPV genotypes were identified in 21 (72.4%) out 29 HPV-positive VSCC, including 7 single HR HPV, 7 multiple HR HPV and 7 HR HPV with other risk-class genotypes. Single or multiple low-risk (LR) HPV genotypes were detected in 8 cases (27.6%). Multiple HPV-genotypes infections were identified in 16 HPV-positive VSCC (55.2%). In our study population, HPV 16 (44.8%), HPV 52 (27.6%) and HPV 44 (24.1%) were the most prevalent genotypes detected, followed by HPV 31 (17.2%), HPV 6 (17.2%), HPV 53 (13.8%), HPV 51 (10.3%) and HPV 11 (10.3%). The main socio-demographic, clinical and histo-pathological characteristics of patients enrolled, according to HPV status, are reported in Table 1.
Table 1: Socio-demographic, clinical and histopathologic characteristics of patients affected by VSCC according to HPV status
|
|
HPV-negative VSSC N=27 n (%) |
HPV-positive VSCC N=29 n (%) |
p-value* |
|
Age |
|
|
0.007 |
|
< 75 years |
9 (33.3) |
21 (72.4) |
|
|
≥ 75 years |
18 (66.7) |
8 (27.6) |
|
|
Menopause |
25 (92.6) |
22 (75.9) |
0.146 |
|
Smoking |
2 (7.4) |
5 (17.2) |
0.424 |
|
ASA score |
|
|
0.382 |
|
≤ 2 |
21 (77.8) |
19 (65.5) |
|
|
> 2 |
6 (22.2) |
10 (34.5) |
|
|
Immunosuppression |
0 (0.0) |
9 (31.0) |
0.002 |
|
Associated vulvar disease |
|
|
< 0.001 |
|
Lichen sclerosus |
16 (59.3) |
10 (34.5) |
|
|
uVIN |
0 (0.0) |
11 (37.9) |
|
|
dVIN |
9 (33.3) |
5 (17.2) |
|
|
Tumour size ≥ 2 cm |
23 (85.2) |
22 (75.9) |
0.506 |
|
Tumour location |
|
|
0.285 |
|
Middle/Bi-lateral |
16 (59.3) |
12 (41.4) |
|
|
Uni-lateral |
11 (40.7) |
17 (58.6) |
|
|
Ulceration |
21 (77.8) |
17 (58.6) |
0.158 |
|
Stromal infiltration > 1 mm |
25 (92.6) |
22 (75.9) |
0.146 |
|
Grading G3 |
8 (29.6) |
8 (27.6) |
1.000 |
|
Type of surgery |
|
|
0.236 |
|
Wide local excision/Emi-vulvectomy |
4 (14.8) |
10 (34.5) |
|
|
Simple vulvectomy |
3 (11.1) |
2 (6.9) |
|
|
Radical vulvectomy |
20 (74.1) |
17 (58.6) |
|
|
Positive surgical margins |
3 (11.1) |
7 (24.1) |
0.299 |
|
Sentinel node biopsy |
11 (40.7) |
10 (34.5) |
0.462 |
|
Positive† |
5 (45.5) |
3 (30.0) |
|
|
Lymphadenectomy |
|
|
0.008 |
|
Inguinal |
21 (77.8) |
11 (37.9) |
|
|
Inguinal + pelvic |
1 (3.7) |
4 (13.8) |
|
|
Lymph node metastasis |
8 (29.6) |
8 (27.6) |
1.000 |
|
Lymph node extracapsular spread |
4 (14.8) |
3 (10.3) |
0.918 |
|
Distant metastasis |
1 (3.7) |
1 (3.4) |
1.000 |
|
FIGO stage |
|
|
0.783 |
|
I |
16 (59.3) |
19 (65.5) |
|
|
II |
2 (7.4) |
1 (3.4) |
|
|
III |
8 (29.6) |
7 (24.1) |
|
|
IV |
1 (3.7) |
2 (6.9) |
|
|
Adjuvant radiotherapy |
8 (29.6) |
4 (13.8) |
0.199 |
|
Adjuvant chemotherapy |
1 (3.7) |
1 (3.4) |
1.000 |
VSCC = vulvar squamous cell carcinoma HPV = Human Papillomavirus
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasia
dVIN = differentiated vulvar intraepithelial neoplasia FIGO = International Federation of Obstetrics and Gynecologists
* Fisher’s Exact Test † Percentage out of performed sentinel node biopsies
Women affected by HPV-positive VSCC were significantly younger (mean age: 64.2 ± 13.4 years; range:32-84) than HPV-negative VSCC patients (mean age: 75.9 ± 10.5 years; range: 43-92) (p=0.007). Nine patients out of 56 (16.1%) were immunosuppressed and all were affected by HPV-positive VSCC (p=0.002). Lichen sclerosus (59.3%) and differentiated VIN (dVIN) (33.3%) were mostly identified in HPV-negative VSCC pathological specimens, while HPV-positive VSCC were more frequently close to usual VIN (uVIN) (37.9%) and all these associations resulted statistically significant (p < 0.001). No significant difference was found in disease-specific and treatment-specific characteristics among HPV-negative and HPV-positive VSCCs, except for the rate of lymph node dissection. In fact, inguinal lymphadenectomy was performed in 77.8% of HPV-negative and 37.9% of HPV-positive patients, respectively (p=0.008). As shown in Table 2, HR HPV-positive VSCCs were significantly associated to age < 75 years (85.7%, p < 0.001), immunosuppression (38.1%, p < 0.001), uVIN (47.6%, p < 0.001) and lower rate of lymph node dissection (33.3%, p=0.008), as well.
Table 2: Socio-demographic, clinical and histopathologic characteristics of patients affected by HPV-positive VSCC, detailed by HPV class-risk
|
|
HR-HPV VSCC N=21 n (%) |
LR-HPV VSCC N=8 n (%) |
p-value* |
|
Age < 75 years |
18 (85.7) |
3 (37.5) |
< 0.001 |
|
Menopause |
14 (66.7) |
8 (100.0) |
0.035 |
|
Smoking |
4 (19.0) |
1 (12.5) |
0.415 |
|
ASA score > 2 |
7 (33.3) |
3 (37.5) |
0.562 |
|
Immunosuppression |
8 (38.1) |
1 (12.5) |
< 0.001 |
|
uVIN |
10 (47.6) |
1 (12.5) |
< 0.001 |
|
Tumour size ≥ 2 cm |
14 (66.7) |
8 (100.0) |
0.111 |
|
Tumour location |
|
|
0.164 |
|
Middle/Bi-lateral |
7 (33.3) |
5 (62.5) |
|
|
Uni-lateral |
14 (66.7) |
3 (37.5) |
|
|
Ulceration |
10 (47.6) |
7 (87.5) |
0.045 |
|
Stromal infiltration > 1 mm |
14 (66.7) |
8 (100.0) |
0.035 |
|
Grading G3 |
5 (23.8) |
3 (37.5) |
0.721 |
|
Type of surgery |
|
|
0.387 |
|
Wide local excision/Emi-vulvectomy |
8 (38.1) |
2 (25.0) |
|
|
Simple/radical vulvectomy |
13 (61.9) |
6 (75.0) |
|
|
Positive surgical margins |
6 (28.6) |
1 (12.5) |
0.286 |
|
Positive sentinel node biopsy |
1 (4.8) |
2 (25.0) |
0.173 |
|
Lymphadenectomy |
|
|
0.008 |
|
Not performed |
12 (57.1) |
2 (25.0) |
|
|
Inguinal |
7 (33.3) |
4 (50.0) |
|
|
Lymph node metastasis |
4 (19.0) |
4 (50.0) |
0.278 |
|
Lymph node extracapsular spread |
2 (9.5) |
1 (12.5) |
0.419 |
|
Distant metastasis |
0 (0.0) |
1 (12.5) |
0.051 |
|
FIGO stage I |
17 (81.0) |
2 (25.0) |
0.020 |
|
Adjuvant radiotherapy |
2 (9.5) |
2 (25.0) |
0.216 |
|
Adjuvant chemotherapy |
0 (0.0) |
1 (12.5) |
0.404 |
|
Progression/recurrence of disease |
5 (23.8) |
2 (25.0) |
0.652 |
|
Death from any cause |
7 (33.3) |
2 (25.0) |
0.030 |
HPV = Human Papillomavirus VSCC = vulvar squamous cell carcinoma
HR = high-risk LR = low-risk
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasia
FIGO = International Federation of Obstetrics and Gynecologists *Fisher’s Exact Test
Furthermore, HR HPV-positive VSCCs were also statistically related to pre-menopausal women (33.3%, p=0.035), lower percentage of ulceration (47.6%, p=0.045) and stromal infiltration > 1 millimetre (mm) (66.7%, p=0.035) and higher prevalence of FIGO stage I (81.0%, p=0.020). VSCCs associated to multiple HPV genotypes infection were significantly more common in women younger than 75 years (93.8%, p < 0.001) and in immunosuppressed patients (31.3%, p=0.002), respectively. uVIN was more frequently detected in association to multiple HPV-positive (37.5%) as well as to single HPV-positive VSCCs (38.5%), as opposed to HPV-negative VSCCs (0.0%) (p < 0.001). Moreover, the rate of lymph node dissection in multiple HPV-positive (43.8%) and in single HPV-positive VSSCs (30.8%) was significantly lower than in HPV-negative VSCCs (77.8%) (p=0.015). After a mean follow-up period of 35.6 ± 38.2 months in HPV-negative patients and 34.8 ± 29.6 months in HPV-positive patients, 4 (14.8%) and 7 (24.1%) progressions or relapses occurred in HPV-negative and HPV-positive VSSC groups, after a mean time of 20.3 ± 31.2 and 17.3 ± 12.5 months, respectively. However, these results were not proven as statistically significant (p=0.506). Overall, deaths from any cause have been more frequently registered in HPV-negative (66.7%) as opposed to HPV-positive VSCC patients (31.0%, p=0.015), and this difference resulted statistically significant even in comparison with HR HPV-positive (33.3%, p=0.030) and multiple HPV-positive VSCC patients (31.3%, p=0.034), respectively (Table 2). According to the Kaplan–Meier survival curves, no differences were found either in DFS (p=0.221), or in OS (p=0.135) between HPV-positive and HPV-negative patients. The estimated 5-year DFS was 70.5% (95% CI: 53.6%-92.8%) in HPV-positive VSCC and 88.6% (95% CI: 77.2% - 100.0%) in HPV-negative VSCC, whereas the estimated 5-year OS was 53.7% (95% CI: 34.8% - 82.7%) and 36.0% (95% CI: 20.2% - 64.2%) in HPV-positive and HPV-negative patients, respectively (Figure 1). Instead, lymph node metastases were significantly associated both with DFS (p=0.021) and OS (p=0.003). In fact, the estimated 5-year DFS was 85.2% (95% CI: 73.7% - 98.4%) in lymph nodes-negative VSCCs and 64.2% (95% CI: 42.9% - 96.1%) in lymph nodes-positive VSCCs, while the estimated 5-year OS was 51.1% (95% CI: 35.6% - 73.4%) and 21.9% (95% CI: 6.6% - 72.1%) in lymph nodes-negative and -positive patients, respectively (Figure 2).
Figure 1: Klapan-Meier curves for 5-year disease-free survival (A) and 5-year overall survival (B) stratified by HPV status.
Figure 2: Klapan-Meier curves for 5-year disease-free survival (A) and 5-year overall survival (B) stratified by lymph nodes involvement.
Moreover, positive surgical margins were statistically related only to DFS (p=0.005), and FIGO stage I only to OS (p=0.021), respectively (Figure 3). At univariate Cox analysis, immunosuppression (p=0.020), positive surgical margins (p=0.016), lymph node metastasis (p=0.032) and lymph node extracapsular spread (p=0.021) were significantly associated to higher risk of progression/relapse, whereas radical vulvectomy was the only factor related to lower risk (p=0.046). On the contrary, increased mortality was correlated to age ≥ 75 years (p=0.007), positive sentinel node (p=0.018), lymph node metastasis (p=0.005), lymph node extracapsular spread (p=0.025), FIGO stage > I (p=0.049) and adjuvant radiotherapy (p=0.014) (Table 3). In the multivariate analysis, lymph node metastasis (HR=8.51, 95% CI: 1.91 – 37.92; p=0.005) and positive surgical margins (HR=8.01, 95% CI: 2.13 – 30.14; p=0.002) correlated with the risk of disease progression/relapse. In a three variable-analysis, including HPV-positivity, this association was retained but decreased by lymph node metastasis (HR=5.92, 95% CI: 2.16 – 16.23; p < 0.001) and positive surgical margins (HR=3.10, 95% CI: 1.13 – 6.50; p=0.028). On the contrary, HPV-positivity was significantly associated to lower risk of disease progression/relapse (HR=0.31, 95% CI: 0.13 – 0.75; p=0.009). For what concern mortality, at multivariate analysis, a significant association was evidenced only for age ≥ 75 years (HR=4.43, 95% CI: 1.83 – 10.70; p < 0.001), lymph node metastasis (HR=3.93, 95% CI: 1.43 – 10.79; p=0.008) and adjuvant radiotherapy (HR=2.50, 95% CI: 0.97 – 6.47; p=0.059). HPV-positivity showed a significant reducing effect on mortality only in two bivariate analysis, adjusting HPV for lymph node extracapsular spread (HR=0.38, 95% CI: 0.16-0.88; p=0.025), and FIGO stage > I (HR=0.42, 95% CI: 0.19 – 0.95; p=0.037), that, instead, related to higher risk of death for any cause (HR=4.46, 95% CI: 1.51 – 13.19; p=0.007 and HR=2.53, 95% CI: 1.12 – 5.73; p=0.026, respectively).
Figure 3: Klapan-Meier curves for 5-year disease-free survival and 5-year overall survival stratified by surgical margins (A-B) and FIGO stage (C-D).
Discussion
In our retrospective analysis, among 56 women affected by VSSC, diagnosed and treated in a single Institute, HPV-DNA was detected in about 52% of corresponding pathological specimens, through a widely validated HPV-genotyping assay, that shows excellent sensitivity and accuracy on FFPE tissues [16]. The most prevalent genotype in our population was HPV 16, as already reported by several Authors [17, 18]. However, the other more frequent genotypes were HPV 52 and HPV 44, that were never related before to VSCC [4, 19]. This could be the result of changes of HPV genotypes distribution, consequent both to the introduction of new HPV genotypes, due to immigration, and different patterns of exposure, related to social and behavioural variables, as already shown by Dal Bello et al. in Italian women affected by cervical intraepithelial neoplasia (CIN) [20]. We compared two groups of VSCC patients, HPV-dependent and HPV-independent, characterized by a similar sample. In literature, several rates of HPV-positive VSCC have been reported, between 15% and 79%, and this wide range is probably related to different HPV-DNA detection techniques, histologic type of vulvar cancer and geographical areas [6, 21-23]. HPV-positive VSCCs usually affect younger patients, with a higher prevalence of immunosuppression, and are significantly associated to uVIN [2, 24, 25]. All these evidences were confirmed in our population study, also when considering HR HPV and multiple HPV genotypes infections, as well as in HPV-related CIN [26]. Our study could be considered as an original analysis because it explored disease- and treatment-specific characteristics of VSCC not only in relation to HPV infection but also to HPV class-risk. According to our analysis, HR HPV-positive VSCCs were significantly associated to lower rate of ulceration and stromal invasion and appeared as FIGO stage I in more than 80% of cases, therefore not requiring lymph node dissection in about 57% of tumours. Other common but not statistically significant features of this group of VSCCs included tumour size < 20 mm, unilateral lesion and lower incidence of lymph node metastasis, with subsequent lower need of adjuvant radiotherapy. This could explain the significantly higher rate of mortality for any cause in HPV-negative VSCC patients, that were usually older at the time of diagnosis and showed more aggressive disease-specific characteristics, especially a higher prevalence of lymph node metastasis and FIGO stage > I. Instead, a higher but not statistically significant incidence of progression/relapse was found in HPV-dependent VSCC, probably related to higher rate of positive surgical margins. Nevertheless, HPV-positive VSCC patients were characterized by lower overall mortality, probably because of less aggressive disease-specific features and subsequent more successful treatment of relapse. In our cohort it was not possible to investigate any correlation between better prognosis of HPV-dependent VSCCs and relevance of radiotherapy for control of the disease, as shown for HPV-positive head and neck tumours, since all relapses of HPV-positive tumours were successfully treated with surgical excision [11, 12]. On the contrary, in 2016 Lee et al. demonstrated that the presence of HPV was significantly related to better 5-year PFS and OS and lower 5-year rate of in-radiation field relapse, but this retrospective analysis included only patients with VSCC who received radiotherapy with or without surgical resection [19].
Table 3: Factors influencing disease progression/recurrence and mortality for any cause at univariate Cox analysis.
|
|
Disease progression/recurrence |
Mortality for any cause |
||
|
|
HR [95% CI] |
p-value* |
HR [95% CI] |
p-value* |
|
Age ≥ 75 years |
2.33 [0.68 – 7.98] |
0.177 |
3.00 [1.34 – 6.71] |
0.007 |
|
Menopause |
1.83 [0.23 – 14.38] |
0.566 |
2.31e+08 [0.00 – Inf] |
0.998 |
|
Smoking |
0.69 [0.09 – 5.41] |
0.726 |
4.38e-09 [0.00 – Inf] |
0.998 |
|
ASA score > 2 |
1.02 [0.27 – 3.88] |
0.978 |
0.96 [0.38 – 2.42] |
0.936 |
|
Immunosuppression |
4.65 [1.28 – 16.96] |
0.020 |
0.65 [0.15 – 2.79] |
0.566 |
|
uVIN |
3.78 [0.93 – 15.35] |
0.063 |
0.46 [0.11 – 2.02] |
0.305 |
|
dVIN |
1.66 [0.37 – 7.46] |
0.507 |
1.15 [0.49 – 2.70] |
0.740 |
|
Tumour size ≥ 2 cm |
1.31 [0.28 – 6.11] |
0.728 |
2.44 [0.73 – 8.13] |
0.146 |
|
Uni-lateral tumour |
0.78 [0.24 – 2.58] |
0.691 |
0.70 [0.33 – 1.49] |
0.354 |
|
Ulceration |
1.34 [0.36 – 5.07] |
0.665 |
1.53 [0.65 – 3.64] |
0.331 |
|
Stromal infiltration > 1 mm |
2.40e+08 [0.00 – Inf] |
0.999 |
2.63 [0.62 – 11.13] |
0.189 |
|
Grading G3 |
1.10 [0.29 – 4.21] |
0.885 |
2.07 [0.95 – 4.50] |
0.066 |
|
Radical vulvectomy |
0.28 [0.08 – 0.98] |
0.046 |
1.20 [0.41 – 3.55] |
0.738 |
|
Positive surgical margins |
4.30 [1.31 – 14.15] |
0.016 |
1.63 [0.65 – 4.10] |
0.297 |
|
Positive sentinel node biopsy |
0.97 [0.12 – 7.91] |
0.976 |
4.35 [1.28 – 14.75] |
0.018 |
|
Inguinal lymphadenectomy |
0.42 [0.11 – 1.58] |
0.199 |
0.77 [0.34 – 1.71] |
0.515 |
|
Pelvic lymphadenectomy |
2.21 [0.42 – 11.57] |
0.349 |
0.61 [0.08 – 4.81] |
0.637 |
|
Lymph node metastasis |
4.28 [1.13 – 16.18] |
0.032 |
3.42 [1.45 – 8.04] |
0.005 |
|
Lymph node extracapsular spread |
5.66 [1.30 – 24.73] |
0.021 |
3.20 [1.15 – 8.85] |
0.025 |
|
Distant metastasis |
2.53 [0.32 – 20.01] |
0.379 |
2.17 [0.51 – 9.32] |
0.296 |
|
FIGO stage > I |
2.10 [0.61 – 7.19] |
0.237 |
2.21 [1.00 – 4.85] |
0.049 |
|
HPV infection |
1.71 [0.50 – 5.84] |
0.392 |
0.47 [0.21 – 1.05] |
0.066 |
|
HR-HPV infection |
1.68 [0.45 – 6.27] |
0.442 |
0.50 [0.21 – 1.22] |
0.128 |
|
Multiple HPV infection |
1.29 [0.29 – 5.80] |
0.739 |
0.45 [0.16 – 1.21] |
0.113 |
|
Adjuvant radiotherapy |
2.91 [0.81 – 10.43] |
0.102 |
2.96 [1.25 – 7.03] |
0.014 |
|
Adjuvant chemotherapy |
4.15 [0.52 – 33.06] |
0.179 |
1.47 [0.20 – 11.01] |
0.705 |
HR = hazard ratio CI = confidence interval
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasiad
VIN = differentiated vulvar intraepithelial neoplasia HPV = Human Papillomavirus
FIGO = International Federation of Obstetrics and Gynecologists HR = high-risk
e = exponential Inf = infinity
* Wald test
In agreement to what reported by previous studies, both DFS and OS were significantly decreased in case of lymph node metastasis, and especially of lymph node extracapsular spread. In addition, positive surgical margins affected the risk of progression/relapse of the disease, whereas age ≥ 75 years, FIGO stage > I and adjuvant radiotherapy increased the risk of mortality for any cause. All these results were statistically significant both at univariate and multivariate Cox regression analysis and confirmed which are the well-known prognostic factors of VSCC [7, 27]. Several Authors have already investigated the existing relationship between HPV infection and prognosis of VSCC, showing opposing results. In 2011, Alonso et al. highlighted that HPV-positivity could not be considered as an independent prognostic factor, as evidenced by our study [6]. Other studies have recently shown a better prognosis related to HPV-positive VSCCs, but in most cases they did not supply the method applied for HPV detection [28]. A retrospective chart review of patients with VSCC stage I showed that HPV-positive and p16-positive patients were less likely to recur and had no disease-specific deaths, thus suggesting a less aggressive type of VSCC related to HPV infection. However, the sample size of available biomarker data was limited, p values were all large (p≥0.25) and multivariate analyses to control for confounding factors could not be applied [28]. Also, Tringler et al. reported a better prognosis related to p16INK4a-positive tumours and van de Nieuwenhof et al. found an increased survival in patients showing uVIN next to VSCC [21, 29]. Although p16INK4a expression and uVIN are notoriously associated to HPV-positivity, they could not be considered as conclusive markers of HPV infection. More recently, Hinten et al. showed that Disease-specific survival (DSS), DFS and OS were significantly better in HPV-related VSCC patients in a large cohort of patients [30]. It is noteworthy that VSCC was considered as HPV-related VSCC in case of >25% p16INK4a expression and HPV presence and even if >25% p16INK4a expression, HPV absence, but uVIN presence. Moreover, only 17% of VSSCs were HPV-related, that is a lower percentage than reported by previous studies [2].
In the end, we could not find a significant association between HPV infection, especially HR HPV and multiple genotypes infection, and risk of progression/relapse of disease and mortality for any cause. However, in our cohort, multivariate regression analysis showed a relevant protective effect of HPV-positivity on risk of disease progression/relapse, in case of concomitant lymph node metastasis and positive surgical margins, and overall mortality in two bivariate analysis, adjusting HPV for lymph node extracapsular spread and FIGO stage > I, respectively. The main weaknesses of our study include bias related to its retrospective single-Institute nature and the small sample size. However, VSCC represents a rare neoplasia of the female genital tract, and the number of enrolled subjects is similar to other single-center studies [31]. In conclusion, no significant association has been proven between HPV infection and risk of progression and overall mortality, even though several clinic and histologic characteristics of VSCC have been related to HR HPV infection. Especially, risk of progression is reduced in HPV-positive patients with node metastasis and positive surgical margins. In addition, HPV infection has a protective effect on overall mortality in case of node extracapsular spread or FIGO stage > I. Nevertheless, multicentre studies with broad sample size are needed to confirm this evidence that could lead to a tailored management of HPV-related VSCCs.
Funding
All Authors declare that there was not involvement of any funding source.
Declaration Of Interests
All Authors declare no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 29, Jan 2019Accepted: Sat 16, Feb 2019
Published: Mon 25, Feb 2019
Copyright
© 2023 Anna Daniela Iacobone. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2019.01.003
Author Info
Arsenio Spinillo Anna Daniela Iacobone Antonietta Mira Barbara Gardella Fabio Bottari Fabio Landoni Paola Alberizzi Stefania Cesari Stefano Bogliolo
Corresponding Author
Anna Daniela IacobonePreventive Gynecology Unit, Division of Gynecology, European Institute of Oncology IRCCS, Via Ripamonti, 435, 20141, Milan, Italy
Figures & Tables
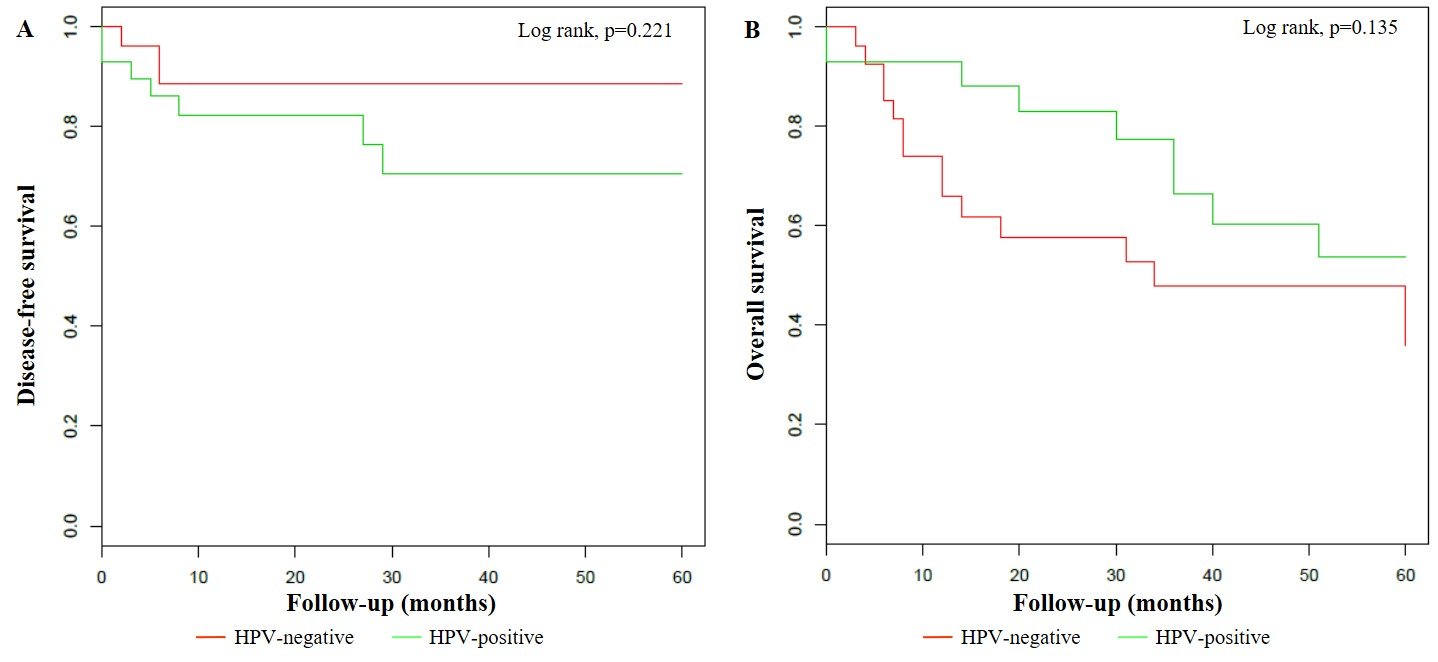
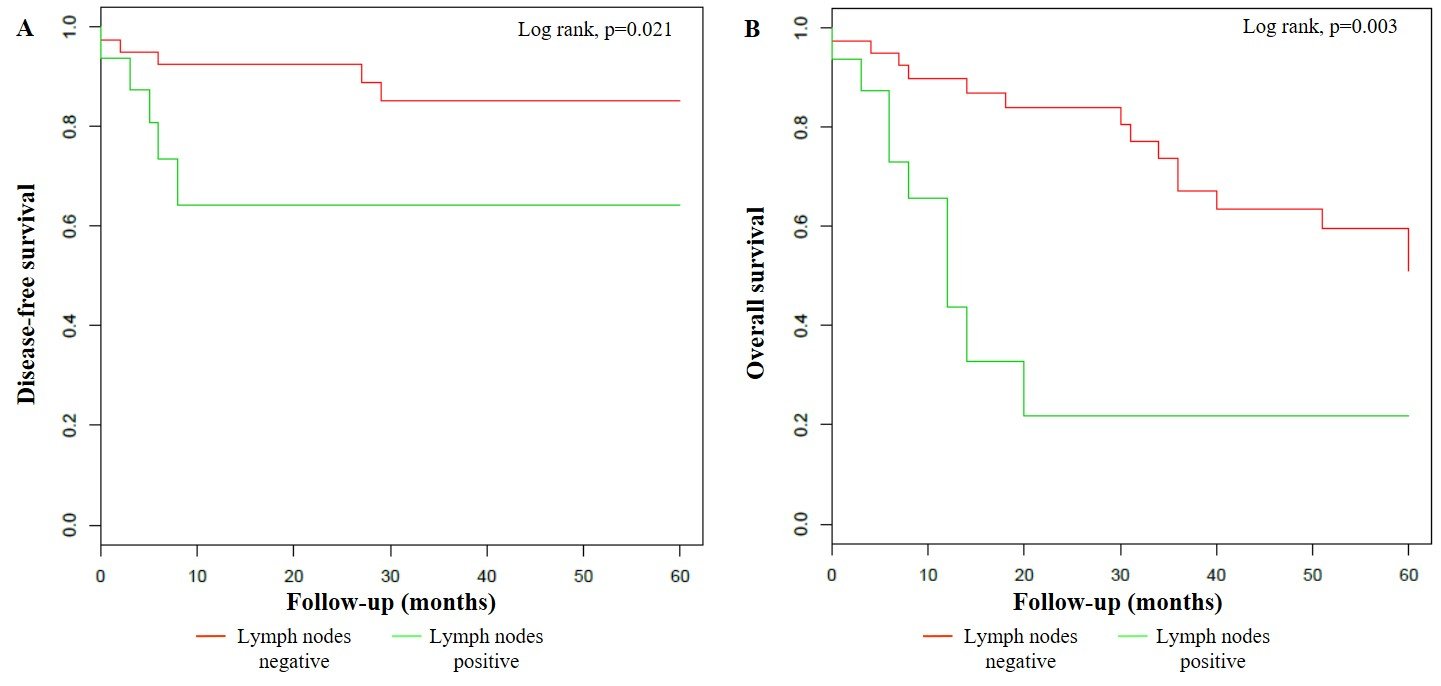
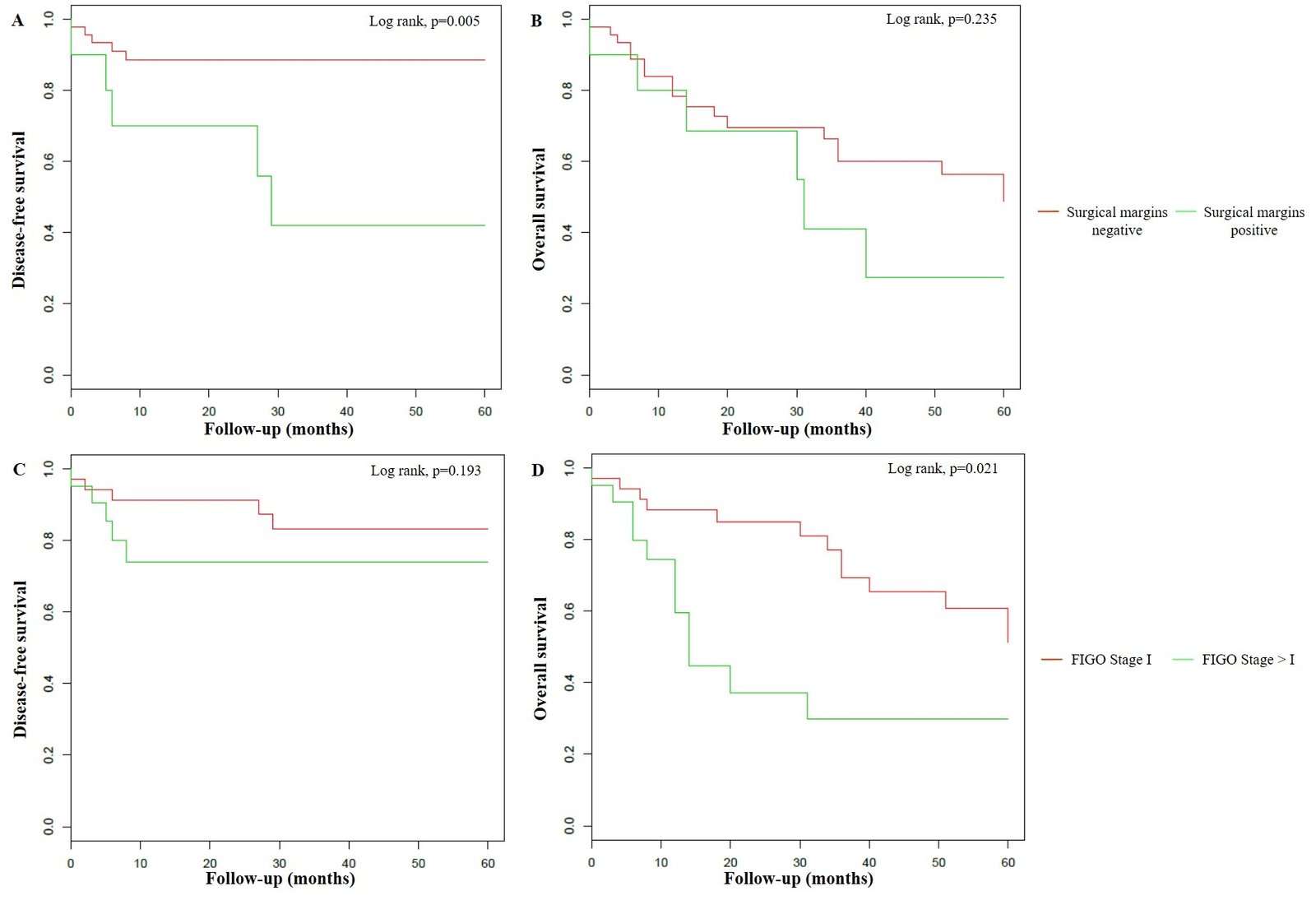
Table 1: Socio-demographic, clinical and histopathologic characteristics of patients affected by VSCC according to HPV status
|
|
HPV-negative VSSC N=27 n (%) |
HPV-positive VSCC N=29 n (%) |
p-value* |
|
Age |
|
|
0.007 |
|
< 75 years |
9 (33.3) |
21 (72.4) |
|
|
≥ 75 years |
18 (66.7) |
8 (27.6) |
|
|
Menopause |
25 (92.6) |
22 (75.9) |
0.146 |
|
Smoking |
2 (7.4) |
5 (17.2) |
0.424 |
|
ASA score |
|
|
0.382 |
|
≤ 2 |
21 (77.8) |
19 (65.5) |
|
|
> 2 |
6 (22.2) |
10 (34.5) |
|
|
Immunosuppression |
0 (0.0) |
9 (31.0) |
0.002 |
|
Associated vulvar disease |
|
|
< 0.001 |
|
Lichen sclerosus |
16 (59.3) |
10 (34.5) |
|
|
uVIN |
0 (0.0) |
11 (37.9) |
|
|
dVIN |
9 (33.3) |
5 (17.2) |
|
|
Tumour size ≥ 2 cm |
23 (85.2) |
22 (75.9) |
0.506 |
|
Tumour location |
|
|
0.285 |
|
Middle/Bi-lateral |
16 (59.3) |
12 (41.4) |
|
|
Uni-lateral |
11 (40.7) |
17 (58.6) |
|
|
Ulceration |
21 (77.8) |
17 (58.6) |
0.158 |
|
Stromal infiltration > 1 mm |
25 (92.6) |
22 (75.9) |
0.146 |
|
Grading G3 |
8 (29.6) |
8 (27.6) |
1.000 |
|
Type of surgery |
|
|
0.236 |
|
Wide local excision/Emi-vulvectomy |
4 (14.8) |
10 (34.5) |
|
|
Simple vulvectomy |
3 (11.1) |
2 (6.9) |
|
|
Radical vulvectomy |
20 (74.1) |
17 (58.6) |
|
|
Positive surgical margins |
3 (11.1) |
7 (24.1) |
0.299 |
|
Sentinel node biopsy |
11 (40.7) |
10 (34.5) |
0.462 |
|
Positive† |
5 (45.5) |
3 (30.0) |
|
|
Lymphadenectomy |
|
|
0.008 |
|
Inguinal |
21 (77.8) |
11 (37.9) |
|
|
Inguinal + pelvic |
1 (3.7) |
4 (13.8) |
|
|
Lymph node metastasis |
8 (29.6) |
8 (27.6) |
1.000 |
|
Lymph node extracapsular spread |
4 (14.8) |
3 (10.3) |
0.918 |
|
Distant metastasis |
1 (3.7) |
1 (3.4) |
1.000 |
|
FIGO stage |
|
|
0.783 |
|
I |
16 (59.3) |
19 (65.5) |
|
|
II |
2 (7.4) |
1 (3.4) |
|
|
III |
8 (29.6) |
7 (24.1) |
|
|
IV |
1 (3.7) |
2 (6.9) |
|
|
Adjuvant radiotherapy |
8 (29.6) |
4 (13.8) |
0.199 |
|
Adjuvant chemotherapy |
1 (3.7) |
1 (3.4) |
1.000 |
VSCC = vulvar squamous cell carcinoma HPV = Human Papillomavirus
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasia
dVIN = differentiated vulvar intraepithelial neoplasia FIGO = International Federation of Obstetrics and Gynecologists
* Fisher’s Exact Test † Percentage out of performed sentinel node biopsies
Table 2: Socio-demographic, clinical and histopathologic characteristics of patients affected by HPV-positive VSCC, detailed by HPV class-risk
|
|
HR-HPV VSCC N=21 n (%) |
LR-HPV VSCC N=8 n (%) |
p-value* |
|
Age < 75 years |
18 (85.7) |
3 (37.5) |
< 0.001 |
|
Menopause |
14 (66.7) |
8 (100.0) |
0.035 |
|
Smoking |
4 (19.0) |
1 (12.5) |
0.415 |
|
ASA score > 2 |
7 (33.3) |
3 (37.5) |
0.562 |
|
Immunosuppression |
8 (38.1) |
1 (12.5) |
< 0.001 |
|
uVIN |
10 (47.6) |
1 (12.5) |
< 0.001 |
|
Tumour size ≥ 2 cm |
14 (66.7) |
8 (100.0) |
0.111 |
|
Tumour location |
|
|
0.164 |
|
Middle/Bi-lateral |
7 (33.3) |
5 (62.5) |
|
|
Uni-lateral |
14 (66.7) |
3 (37.5) |
|
|
Ulceration |
10 (47.6) |
7 (87.5) |
0.045 |
|
Stromal infiltration > 1 mm |
14 (66.7) |
8 (100.0) |
0.035 |
|
Grading G3 |
5 (23.8) |
3 (37.5) |
0.721 |
|
Type of surgery |
|
|
0.387 |
|
Wide local excision/Emi-vulvectomy |
8 (38.1) |
2 (25.0) |
|
|
Simple/radical vulvectomy |
13 (61.9) |
6 (75.0) |
|
|
Positive surgical margins |
6 (28.6) |
1 (12.5) |
0.286 |
|
Positive sentinel node biopsy |
1 (4.8) |
2 (25.0) |
0.173 |
|
Lymphadenectomy |
|
|
0.008 |
|
Not performed |
12 (57.1) |
2 (25.0) |
|
|
Inguinal |
7 (33.3) |
4 (50.0) |
|
|
Lymph node metastasis |
4 (19.0) |
4 (50.0) |
0.278 |
|
Lymph node extracapsular spread |
2 (9.5) |
1 (12.5) |
0.419 |
|
Distant metastasis |
0 (0.0) |
1 (12.5) |
0.051 |
|
FIGO stage I |
17 (81.0) |
2 (25.0) |
0.020 |
|
Adjuvant radiotherapy |
2 (9.5) |
2 (25.0) |
0.216 |
|
Adjuvant chemotherapy |
0 (0.0) |
1 (12.5) |
0.404 |
|
Progression/recurrence of disease |
5 (23.8) |
2 (25.0) |
0.652 |
|
Death from any cause |
7 (33.3) |
2 (25.0) |
0.030 |
HPV = Human Papillomavirus VSCC = vulvar squamous cell carcinoma
HR = high-risk LR = low-risk
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasia
FIGO = International Federation of Obstetrics and Gynecologists *Fisher’s Exact Test
Table 3: Factors influencing disease progression/recurrence and mortality for any cause at univariate Cox analysis.
|
|
Disease progression/recurrence |
Mortality for any cause |
||
|
|
HR [95% CI] |
p-value* |
HR [95% CI] |
p-value* |
|
Age ≥ 75 years |
2.33 [0.68 – 7.98] |
0.177 |
3.00 [1.34 – 6.71] |
0.007 |
|
Menopause |
1.83 [0.23 – 14.38] |
0.566 |
2.31e+08 [0.00 – Inf] |
0.998 |
|
Smoking |
0.69 [0.09 – 5.41] |
0.726 |
4.38e-09 [0.00 – Inf] |
0.998 |
|
ASA score > 2 |
1.02 [0.27 – 3.88] |
0.978 |
0.96 [0.38 – 2.42] |
0.936 |
|
Immunosuppression |
4.65 [1.28 – 16.96] |
0.020 |
0.65 [0.15 – 2.79] |
0.566 |
|
uVIN |
3.78 [0.93 – 15.35] |
0.063 |
0.46 [0.11 – 2.02] |
0.305 |
|
dVIN |
1.66 [0.37 – 7.46] |
0.507 |
1.15 [0.49 – 2.70] |
0.740 |
|
Tumour size ≥ 2 cm |
1.31 [0.28 – 6.11] |
0.728 |
2.44 [0.73 – 8.13] |
0.146 |
|
Uni-lateral tumour |
0.78 [0.24 – 2.58] |
0.691 |
0.70 [0.33 – 1.49] |
0.354 |
|
Ulceration |
1.34 [0.36 – 5.07] |
0.665 |
1.53 [0.65 – 3.64] |
0.331 |
|
Stromal infiltration > 1 mm |
2.40e+08 [0.00 – Inf] |
0.999 |
2.63 [0.62 – 11.13] |
0.189 |
|
Grading G3 |
1.10 [0.29 – 4.21] |
0.885 |
2.07 [0.95 – 4.50] |
0.066 |
|
Radical vulvectomy |
0.28 [0.08 – 0.98] |
0.046 |
1.20 [0.41 – 3.55] |
0.738 |
|
Positive surgical margins |
4.30 [1.31 – 14.15] |
0.016 |
1.63 [0.65 – 4.10] |
0.297 |
|
Positive sentinel node biopsy |
0.97 [0.12 – 7.91] |
0.976 |
4.35 [1.28 – 14.75] |
0.018 |
|
Inguinal lymphadenectomy |
0.42 [0.11 – 1.58] |
0.199 |
0.77 [0.34 – 1.71] |
0.515 |
|
Pelvic lymphadenectomy |
2.21 [0.42 – 11.57] |
0.349 |
0.61 [0.08 – 4.81] |
0.637 |
|
Lymph node metastasis |
4.28 [1.13 – 16.18] |
0.032 |
3.42 [1.45 – 8.04] |
0.005 |
|
Lymph node extracapsular spread |
5.66 [1.30 – 24.73] |
0.021 |
3.20 [1.15 – 8.85] |
0.025 |
|
Distant metastasis |
2.53 [0.32 – 20.01] |
0.379 |
2.17 [0.51 – 9.32] |
0.296 |
|
FIGO stage > I |
2.10 [0.61 – 7.19] |
0.237 |
2.21 [1.00 – 4.85] |
0.049 |
|
HPV infection |
1.71 [0.50 – 5.84] |
0.392 |
0.47 [0.21 – 1.05] |
0.066 |
|
HR-HPV infection |
1.68 [0.45 – 6.27] |
0.442 |
0.50 [0.21 – 1.22] |
0.128 |
|
Multiple HPV infection |
1.29 [0.29 – 5.80] |
0.739 |
0.45 [0.16 – 1.21] |
0.113 |
|
Adjuvant radiotherapy |
2.91 [0.81 – 10.43] |
0.102 |
2.96 [1.25 – 7.03] |
0.014 |
|
Adjuvant chemotherapy |
4.15 [0.52 – 33.06] |
0.179 |
1.47 [0.20 – 11.01] |
0.705 |
HR = hazard ratio CI = confidence interval
ASA = American Society of Anaesthesiologists uVIN = usual vulvar intraepithelial neoplasiad
VIN = differentiated vulvar intraepithelial neoplasia HPV = Human Papillomavirus
FIGO = International Federation of Obstetrics and Gynecologists HR = high-risk
e = exponential Inf = infinity
* Wald test
References
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9-29. [Crossref]
- van der Avoort IA, Shirango H, Hoevenaars BM, Grefte JM, de Hullu JA, et al. (2006) Vulvar squamous cell carcinoma (VSCC) is a multifactorial disease following two separate and independent pathways. Int J Gynecol Pathol 25: 22-29. [Crossref]
- van de Nieuwenhof HP, Bulten J, Hollema H, Dommerholt RG, Massuger LF, et al. (2011) Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol 24: 297-305. [Crossref]
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, et al. (2012) Global burden of human papillomavirus and related diseases. Vaccine 30: 12-23. [Crossref]
- Monk BJ, Burger RA, Lin F, Parham G, Vasilev SA, et al. (1995) Prognostic significance of human papillomavirus DNA in vulvar carcinoma. Obstet Gynecol 85: 709-715. [Crossref]
- Alonso I, Fusté V, del Pino M, Castillo P, Torné A, et al. (2011) Does human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma? Gynecol Oncol 122: 509-514. [Crossref]
- Bogani G, Cromi A, Serati M, Uccella S, Donato VD, et al. (2017) Predictors and Patterns of Local, Regional, and Distant Failure in Squamous Cell Carcinoma of the Vulva. Am J Clin Oncol 40: 235-240. [Crossref]
- Pinto AP, Schlecht NF, Pintos J, Kaiano J, Franco EL, et al. (2004) Prognostic significance of lymph node variables and human papillomavirus DNA in invasive vulvar carcinoma. Gynecol Oncol 92: 856-865. [Crossref]
- Lindell G, Näsman A, Jonsson CA, Tina Dalianis, Barbro Larson, et al. (2010) Presence of human papillomavirus (HPV) in vulvar squamous cell carcinoma (VSCC) and sentinel node. Gynecol Oncol 117: 312-316.
- Ansink AC, Krul MR, De Weger RA, Kleyne JA, Pijpers H, et al. (1994) Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: detection and prognostic significance. Gynecol Oncol 52: 180-184. [Crossref]
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261-269. [Crossref]
- Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, et al. (2009) Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27: 1992-1998. [Crossref]
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Høgdall E, Geertsen PF, et al. (2014) Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 32: 1812-1817. [Crossref]
- Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, et al. (2012) Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecol Oncol 127: 147-152. [Crossref]
- Tavassoli FA, Devilee P (2003) Pathology and genetics of tumours of the breast and female genital organs, 1st ed. Lyon: IARC.
- Alberizzi P, Spinillo A, Gardella B, Cesari S, Silini EM (2014) Evaluation of the HPV typing INNO-LiPA EXTRA assay on formalin-fixed paraffin-embedded cervical biopsy samples. J Clin Virol 61: 535-539. [Crossref]
- De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S (2009) Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 124: 1626-1636. [Crossref]
- Srodon M, Stoler MH, Baber GB, Kurman RJ (2006) The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). Am J Surg Pathol 30: 1513-1518. [Crossref]
- Lee LJ, Howitt B, Catalano P, Tanaka C, Murphy R, et al. (2016) Prognostic importance of human papillomavirus (HPV) and p16 positivity in squamous cell carcinoma of the vulva treated with radiotherapy. Gynecol Oncol 142: 293-298. [Crossref]
- Dal Bello B, Spinillo A, Alberizzi P, Cesari S, Gardella B, et al. (2009) Time trends of human papillomavirus type distribution in Italian women with cervical intraepithelial neoplasia (CIN). Gynecol Oncol 115: 262-266. [Crossref]
- van de Nieuwenhof HP, van Kempen LC, de Hullu JA, Bekkers RL, Bulten J, et al. (2009) The etiologic role of HPV in vulvar squamous cell carcinoma fine tuned. Cancer Epidemiol Biomarkers Prev 18: 2061-2067. [Crossref]
- Gargano JW, Wilkinson EJ, Unger ER, Steinau M, Watson M, et al. (2012) Prevalence of human papillomavirus types in invasive vulvar cancers and vulvar intraepithelial neoplasia 3 in the United States before vaccine introduction. J Low Genit Tract Dis 16: 471-479. [Crossref]
- Kowalewska M, Szkoda MT, Radziszewski J, Ptaszynski K, Bidzinski M, et al. (2010) The frequency of human papillomavirus infection in polish patients with vulvar squamous cell carcinoma. Int J Gynecol Cancer 20: 434-437. [Crossref]
- Al-Ghamdi A, Freedman D, Miller D, Poh C, Rosin M, et al. (2002) Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol 84: 94-101. [Crossref]
- Pinto AP, Signorello LB, Crum CP, Harlow BL, Abrão F, et al. (1999) Squamous cell carcinoma of the vulva in Brazil: prognostic importance of host and viral variables. Gynecol Oncol 74: 61-67. [Crossref]
- Bello BD, Spinillo A, Alberizzi P, Cesari S, Gardella B, et al. (2009) Cervical infections by multiple human papillomavirus (HPV) genotypes: Prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol 81: 703-712. [Crossref]
- Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, et al. (2007) Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol 104: 636-641. [Crossref]
- Hay CM, Lachance JA, Lucas FL, Smith KA, Jones MA (2016) Biomarkers p16, Human Papillomavirus and p53 Predict Recurrence and Survival in Early Stage Squamous Cell Carcinoma of the Vulva. J Low Genit Tract Dis 20: 252-256. [Crossref]
- Tringler B, Grimm C, Dudek G, Zeillinger R, Tempfer C, et al. (2007) p16INK4a expression in invasive vulvar squamous cell carcinoma. Appl Immunohistochem Mol Morphol 15: 279-283. [Crossref]
- Hinten F, Molijn A, Eckhardt L, Massuger LFAG, Quint W, et al. (2018) Vulvar cancer: Two pathways with different localization and prognosis. Gynecol Oncol 149: 310-317. [Crossref]
- Imoto S, Inamine M, Kudaka W, Nagai Y, Wakayama A, et al. (2016) Prognostic factors in patients with vulvar cancer treated with primary surgery: a single-center experience. Springerplus 5: 125. [Crossref]
