Journals
The Presence of Biofilms in Gouty Tophi
A B S T R A C T
In patients who had confirmed gout, we have pathologically identified biofilms in cutaneous tophi. Clinically, these lesions were asymptomatic, off-white papules on the ears and/or extremities. The methods used for confirmation were the same as those used for biofilms in other organs, namely staining for the extracellular polysaccharides which make up the bulk of the biofilm with colloidal iron (CFE), and Congo red (CR) that stains the amyloid that serves as infrastructure of the biomass. CFE was used, instead of periodic acid Schiff (PAS), because of the presence of uric acid crystals in the tophi; CFE, which stains acidic mucin, was used and showed more positivity than PAS that stains neutral mucins. These stains were positive in the areas of the tophus and negative in the surrounding tissue. CD 282 (Toll-like receptor 2) immunostain was negative in all, save for faint staining in one specimen, indicating the immune system was not involved.
K E Y W O R D S
HIV, ART, Adherence, PMTCT, Social support
I N T R O D U C T I O N
In prior studies, we, and others, have found biofilms created by many different organisms, in many different organs, and in many different chronic diseases [1-8]. These have ranged from staphylococcal biofilms occluding sweat ducts in eczema to streptococcal biofilms in the tonsils in psoriasis [1, 2]. Also, we have commented on biofilms in chronic internal diseases like those from spirochetes in Alzheimer’s disease (AD) that lead to disease locally , and in arteriosclerotic arteries where the disease may be local (thrombosis) or distal (embolism) [7, 8].
Biofilms have been noted in both intra and extracellular locations. In eczema and tinea versicolor, they are exclusively extracellular; and in molluscum and squamous cell carcinoma in situ, they are only (and can be only) intracellular because of their viral nature [1, 3, 9, 10]. In psoriasis and AD, they are both intra and extracellular [2, 7]. With the above as background, we have examined skin specimens from patients with gout for the presence of biofilms in what had previously been considered solely a deposit of uric acid crystals [11].
Methods
Ten specimens from 7 M and 3 F ages 58-82 were initially diagnosed as gout (tophus) with routine hematoxylin and eosin histological staining. Colloidal iron CFE (for acidic mucins) and Congo red stains were also performed on these specimens as was CD 282 immunostain. The findings were confirmed by 4 dermatopathologists. As controls, 10 specimens from healing wounds were examined, except for CD 282.
Results
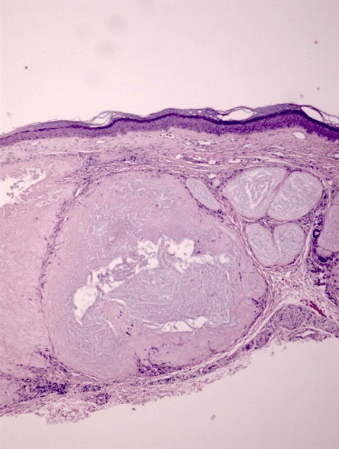
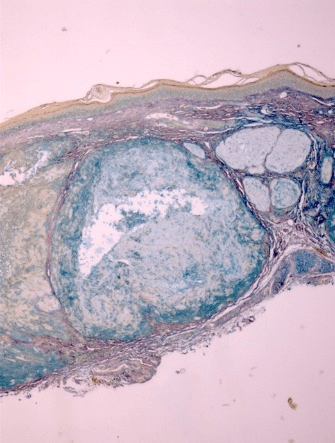
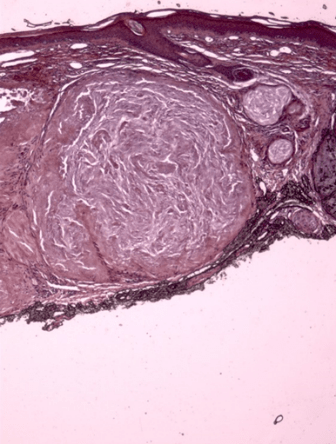
All ten specimens showed focally positive intradermal staining with colloidal iron (CFE) and Congo red (CR) in the area of the tophus (Figure 1-3). The ten specimens of healing wounds were negative for similar dermal staining. The staining occurred in the areas of the uric acid depositions and indicate that biofilms are involved in the disease process. There was faint staining with CD282 in one specimen and all the rest were negative. This indicates that TLR2 was minimally involved.
Figure 1: H+E stain of cutaneous tophus; an accumulation of uric acid is noted in the dermis. 10X
Figure 2: Positive staining of same tophus with colloidal iron, indicating acidic mucin. 10X
Figure 3: Same tophus stains with Congo Red indicating the presence of amyloid. 10X
Discussion
All ten of our patients had clinically confirmed gout with elevated serum uric acid in conjunction with chronic arthritis, and occasional flares of acute arthritis, in focal joints. These patients presented dermatologically with asymptomatic small skin deposits which on routine histologic examination revealed focal deposition of an amorphous material which has previously been shown to be uric acid crystals [11]. In the present study, we have confirmed the presence of biofilms in those areas of uric acid deposition. These biofilms were confirmed pathologically by positive CFE and CR staining which stain the mucin and amyloid in the similar biofilms that have been found in the study of other skin diseases such as eczema and tinea versicolor [1, 3].
Further, these biofilms did not stain for CD282 except for faint staining in one specimen indicating the lack of immune system involvement. This fits with the clinical finding that these lesions were asymptomatic and presented simply as “new growths.” In eczema, where the intraductal biofilms attracted TLR2, we have shown that the prominent symptomatic response, the marked pruritus, is due to the TLR2 upregulating the known pruritogen, PAR2, to cause the unrelenting itching. The TLR2 leaves its control location in the basal layer to surround the affected sweat ducts in the upper epidermis in eczema [1].
Where there are biofilms, there are microbes, and where there are chronic diseases, especially chronic diseases with depositions, there is a strong possibility of the presence of biofilms and the presence of the microbes that make them. These have been shown in all types of arthritis, in many skin diseases, and in many systemic diseases [1-9, 11]. In the deposition diseases, the biofilms are extracellular because there are few, if any cells, in the depositions. In other diseases, particularly viral diseases, the biofilms must be intracellular, because the virus needs the cellular DNA to create the biofilms. In other diseases, such as psoriasis and AD, the biofilms have been found in both intra and extracellular locations. Moreover, the intracellular biofilms do not attract the immune system.
In this work, we have not identified any microbes; that will be for a subsequent effort. Some of the microbes that certainly will require attentions will be dental organisms. They have been shown to play a role in other chronic diseases with both direct and epidemiological findings [7, 11].
Article Info
Article Type
Research ArticlePublication history
Received: Wed 06, Feb 2019Accepted: Fri 01, Mar 2019
Published: Mon 18, Mar 2019
Copyright
© 2023 Herbert B. Allen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2018.01.007
Author Info
Carrie Ann Cusack Herbert B. Allen Robert A Allen
Corresponding Author
Herbert B. AllenDrexel University College of Medicine, Department of Dermatology
References
- Allen HB, Vaze ND, Choi C, Hailu T, Tulbert BH, et al. (2014) The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 150: 260-265. [Crossref]
- Allen HB, Jadeja S, Allawh RM, Goyal K (2018) Psoriasis, chronic tonsillitis, and biofilms: Tonsillar pathologic findings supporting a microbial hypothesis. Ear Nose Throat J 97: 79-82. [Crossref]
- Allen HB, Goyal K, Ogrich L, Joshi S (2015) Biofilm formation by Malassezia furfur/ovale as a possible mechanism of pathogenesis in Tinea versicolor. J Clin Exp Dermatol Res 6: 311.
- Allen HB, Moschella SL (2017) The Role of Rifampin in Leprosy: Leprosy Through a New Lens. JAMA Dermatol 153: 261-262. [Crossref]
- Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, et al. (2012) Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am 94: 2247-2254. [Crossref]
- Allen HB, Heffner B, Dasgupta T, Cusack CA, Sen B, et al. (2016) Pruritus of Healing Wounds: why “Scabs” Itch. J Clin Exp Dermatol Res.
- Allen HB (2016) Alzheimer’s disease: Assessing the Role of Spirochetes, Biofilms, the Immune System, and Beta Amyloid with regard to potential Treatment and Prevention. J Alz Dis 53: 1271-1276. [Crossref]
- Allen HB, Boles J, Morales D, Ballal S, Joshi SG (2016) Arteriosclerosis: The Novel Finding of Biofilms and Innate Immune System Activity within the Plaques. J Med Surg Pathol 1: 135.
- Allen HB, Allawh RM, Ballal S (2017) Virally-Induced, Intracellular Biofilms; Novel Findings in Molluscum Contagiosum. Clin Microbiol 6: 302.
- Allen HB, Chung CL, Allawh RM, Larijani M, Cusack CA (2019) Clin Dermatol and Dermatitis in press.
- Ragab G, Elshahaly M, Bardin T (2017) Gout: An old disease in new perspective – A review. J Adv Res 8: 495-511. [Crossref]
