The Science behind Autologous Fat Grafting. An Institutional Retrospective Review and the Current State
A B S T R A C T
Aim: Using adipose tissue transfer to correct facial and maxillo-facial defects was first reported at the end of the 19th century. Afterwards, autologous fat grafting was launched as a way of improving facial aesthetics. More recently, it has expanded into additional applications in cranio-maxillofacial and reconstructive surgery. Several approaches have been suggested for harvesting, treating, and grafting the fat. Nonetheless, because many adipocytes are unavoidably damaged during these maneuvers, the results have not always been as desired and have therefore needed several sessions of fat injection.
Methods: The authors provide an overview of the application of fat grafting in managing volumetric deficit in the craniofacial and maxillofacial areas in patients undergoing long-term follow up. Understanding the fundamental science principles of fat grafting is critical to explain its extensive usefulness for regeneration, rejuvenation, new texture, and soft tissue volumization. The popularity of autologous fat grafts is not only because of its usefulness in volume replacement and improvement in tissue quality but also its low donor-site morbidity and its remarkable levels of accessibility, availability, and biocompatibility. The authors survey the current literature on fat grafting techniques, evaluate the potential benefits in of reconstructive surgery, and discuss recent scientific developments to optimize fat graft survival and outcomes.
Conclusion: This article discusses the biology of adipose tissue and the principles of the behaviour and survival of adipose tissue in an autologous fat grafting laboratory. Clinical data suggest that the use of adult stem cells is a very promising field, and the future therapeutic applications are enormous in different disorders as well as in aesthetic applications.
Keywords
Adipose tissue, adipogenesis, facial reconstruction, tissue repairing, regenerative medicine, tissue engineering, adipose-derived stem cells, fat graft survival, bench to bedside
Introduction
The use of adipose tissue transfer for correcting facial defects was first reported at the end of the 19th century. Some surgeons have used fat grafts since the late 1800s the results of which, however, have been uncertain and largely determined by technique. Many reports in the literature of the early 1900s described tissue augmentation with various injectable fillers such as gold, rubber latex, gutta–percha, paraffin, collagen, goretex, silicon, and more recently, polylactic acid and byoalcamid [1, 2]. In 1991 the New York plastic surgeon Sydney R. Coleman systematized the technique by focusing on the face. This was based on the idea that fat was the ideal filler because it was natural, stable, and without the complications of earlier fillers. Fat grafting (FG) differs from other techniques, because it requires a delicate aspiration to protect the fragile adipocytes, the purification of the material, and its reinsertion through microinjections to redefine facial contours and create a more harmonious and aesthetically appealing symmetry of the face [3, 4]. Human adipose tissue is a rich source of mesenchymal stem cells (Figure 1) which exhibit multilineage potential and secrete angiogenic and antiapoptotic factors [5, 6]. The main indications for FG include restoration, volumization, and soft tissue facial reconstruction after trauma, tumor resection, or congenital deformities and clefts, Parry-Romberg syndrome, scleroderma, orbital and periorbital surgery, facial palsy, burns, and scars [7, 8]. Fat is also used for facial rejuvenation in areas such as the forehead, zygomas, lips, nose, chin, and mandible [9, 10]. Wide procedural variation in FG is thought to contribute to the difference in resorption rates and outcomes. There is still no universal protocol for the best procedure, in spite of considerable investigation into all the steps. To better understand the outcomes, the biological principles of adipose tissue and the mechanisms of its survival have also been extensively investigated. An in-depth understanding of the basic science behind FG is essential to establish the best practice guidelines, facilitate graft survival, and enhance long-term results.
Figure 1: Adipocyte stem-cell under electron microscope.
Materials and Methods
I Procedural Approach: Fat Harvesting, Refinement, Purification, and Placement
Meticulous photography is requisite for successful Coleman fat grafting. Photographs of the full face are taken from many angles to assess proportions, and afterwards closeup photographs are taken to isolate and analyse individual facial areas. The photographs are evaluated with the patient at the second consultation [3]. Various coloured pencils are used on these photographs in the areas to be injected, those not injected, and, if necessary, the areas from which excessive fat tissue should be removed. Such markings are extremely important in the last part of the planning of the operation, for the informed consent, and for guiding the placement of fat grafts. They are duplicated on the patient’s face preoperatively. Common donor sites are the trochanteric region, inner thighs, knees, abdomen and periumbilical area, and flanks. In children, the buttocks are usually the preferred donor area. Surgery is preferably performed with patients under general anaesthesia or local anaesthesia with sedation. Antithrombotic stockings are always advisable. The fat is aspirated using a very thin liposuction cannula attached to a 10-mL Luer Lock syringe.
During the fat removal, care is taken to minimize mechanical trauma to the adipocytes. Once the fat has been removed, every 10 mL syringe of fat is carefully placed into a sterilized sleeve of a centrifuge rotor and spun at approximately 1,300 rpm for three minutes. The fat separates into three layers: the top is primarily composed of oil from ruptured parcels of fat, the middle of usable fat staminal tissue, and the bottom layer of blood. The top oil is decanted, and the dense lowest layer is drained. The refined parcels are then transferred to a 1-mL syringe and layered into the areas that need to be enhanced proceeding from the underlying bone up to the skin surface in a fan-shaped way [11, 12]. Cannulas with different tip shapes, diameters, and lengths can be used. Blunt cannulas permit the placement of fat parcels in a manner that is more stable and less traumatic. However, less blunt cannulas may allow the surgeon to have a better control of this placement in the immediate subdermal layer of fibrous and scar tissue. A cannula with a pointed or sharp tip is used to disentangle adhesions. Fatty tissue should be injected only as the cannula is withdrawn.
With linear deposition, the fat is layered into the area in need of improvement, working from the underlying bone up to the surface of the skin to produce a three-dimensional grill in a fan shape. The largest amount of tissue that should be placed with each withdrawal of a cannula is 1/10 mL, but in some areas, such as the eyelid, the maximum placed should be from 1/30 to 1/50 mL per withdrawal. One-step correction, using more fat tissue, can cause poor vascularization and increased reabsorption, particularly in areas covered by a thin layer of soft tissue like the maxillofacial area [13, 14]. FG allows the fat to be carefully layered into minuscule strands creating a large surface area of contact with the augmented site, ensuring that nutrition can be exchanged through capillaries in the enveloping tissue which is essential for the fat to survive.15 Using the patient’s own fat insures there is no risk of allergic reactions or rejections by the body. The deeply embedded fat does not shift or migrate. When the surgery is over, compression dressings are applied around the infiltrated areas for 3-4 days. If the fat is harvested from the abdomen the patient wears an abdominal binder. Ice packs are applied continuously on all infiltrated sites for 24 to 36 hours postoperatively.
Postoperative care involves use of antibiotics for several days and anti-inflammatory therapy massage of the donor area to alleviate swelling and bruising. Patients should be instructed that postoperative swelling can last for up to 8 to 12 days [16]. Recovery time will depend on the extent of the procedure. The amount of absorption is variable and frequently unpredictable [17, 18]. The percentage of fat reabsorption varies from patient to patient. If a considerable amount of fat is absorbed, a second procedure may be needed to achieve the desired outcome. When dealing with craniofacial deformities, consider that patients have altered growth potential. Fat reabsorption is higher in these patients compared with others, and FG should be done as many as 3 to 5 times to improve the result, with a lapse of 8 to 14 months.
Clinical Cases
The authors present eight different clinical cases which support the indication that the FG technique should be included in the complex process of reconstructive planning and procedures. All patients underwent general anaesthesia. A comparison was made of frontal and three-fourth views, with close-up photos taken preoperatively and during the follow-up (Figures 2-8).
Figure 2: A) Asymmetry of the middle-lower third. B) Chin remodeling, upper maxilla Le Fort 1 osteotomy with advancement, nasal tip revision, extensive FG with rejuvenation and reshaping of the lips.
Figure 3: A) Romberg disease (rare disease) with severe right facial hemiatrophy. B) Facial reconstruction with three FG procedures and regeneration.
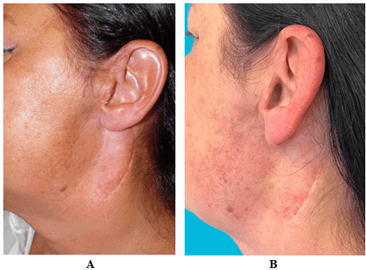
Figure 4: A) Lateral cervical deformity after removal of a benign parotid tumor. B) Two FG procedures were performed with skin volumization and regeneration.
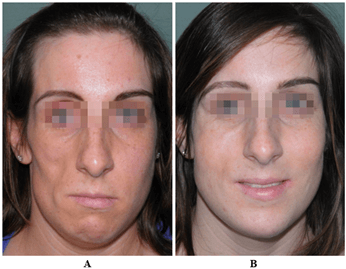
Figure 5: A) Facial asymmetry. B) Total facial remodeling with nasal tip reshaping and otoplasty for protruding ears. Extensive FG with regeneration: cheekbones, forehead, lips and perioral region, reinforcement and contouring of the chin-mandibular lines.
Figure 6: Unilateral right cleft lip and palate sequelae. A) Depression and disharmony of the upper vermilius and of the missing philtrum. B) Three years after two FG procedures with reshaping of the cupid bow and fullness of the perioral area.
Figure 7: Treacher Collins Syndrome, severe congenital craniofacial disease. A) The patient at age 6 before any reconstructive procedure. B) After orbital bony reconstruction: soft tissue skeletonization and deficiency age 13. C) Three FG procedures were performed with improvement of the orbital-zygomatic and temporal areas, age 17.
Figure 8: A) Left facial palsy. B) After blink restoration with transposition of the temporalis muscle flap fascia. Total FG was performed as a later stage with augmentation of facial volumes and reduction of the facial paralysis stigmata.
Results And Discussion
Fat grafting was introduced as a way of enhancing facial aesthetics and in the past few years has expanded into applications in more complex reconstructive procedures [19]. It provides a technique characterized by biocompatibility that easily adapts to the development of the face over time. It has a lower morbidity compared with many other alloplastic materials making FG the ideal tool for the correction of localized tissue atrophy, loss of substance due to trauma, post-tumor and congenital complex craniofacial anomalies, burns, as well as hemifacial atrophy, that is, Parry-Romberg syndrome and scleroderma. Results are natural and enduring [7, 20-22]. Because minimum trauma to the adipocytes is extremely important, different types of needles have been used for harvesting [11]. There are four main ways to purify the adipose tissue: centrifugation, filtration, decantation, and washing. We compared centrifugation with other techniques such as percolator and decantation.
According to Mojallal et al., centrifugation is the best way to purify adipose tissue because there is no deterioration or damage to the adipocytes, and because it increases the fat density by eliminating red blood and cellular debris [11, 13, 23]. With this approach, there are no consequences on the cellular structure, and there are no differences in cellular structures between centrifuged adipose cells and adipose cells that are not centrifuged. Using a blunt cannula and centrifugation, more than 90% of adipocytes survive and have normal enzyme activity [14]. FG is a useful technique in craniofacial and maxillofacial surgery because it can be collected in relatively large quantities with minimal risk. Despite it being a safe procedure, complications may occur. The most frequent complications are overcorrection, under-correction, lumps, bumps, fat migration, infection, damage to underlying structures, and ischaemic necrosis. Fat embolism has been reported in a few cases [24, 25].
Other FG applications include recalcitrant ectropion, anopthtalmic socket, orthognathic surgery, scars or burn wounds, and ectropion (post-trauma, congenital, post different reconstructions). Several techniques have been described and reported, some of which are successful and others are not. The main problem of ectropion is the scarcity of skin, muscles, and internal lamellae. A palatal mucosal graft to rebuild the internal lamella is quite useful but it does not allow restoring the skin and the orbicularis muscle. The stretching of the internal lamella can be combined (in a second stage) with micro-parcels of fat. The result is a lengthening of the lower eyelid with improvement in volume and texture. FG in orthognathic surgery is a recent application. As well known, in orthognathic surgery skeletal bases, maxilla, mandible, and chin are moved and repositioned three-dimensionally. In some dentofacial deformities, soft tissue may be lacking enough to impede improvement by bone repositioning. FG provides a better texture and increases the lightness of the face [7]. Results of various studies have verified that human adipose tissue is a rich source of mesenchymal stem cells (ASCs) which exhibit multilineage potential and enhance the secretion of angiogenic and antiapoptotic factors [5, 6].
Adipose-derived stem cells, which in the past were called either adipose-derived stromal cells or adipose-derived regenerative cells, are isolated from the vascular stromal component of lipoaspirate. A standard raw lipoaspirate consists of mature adipocytes, extracellular matrix, ASCs, endothelial cells, and mural cells (pericytes and vascular smooth muscle cells). The enzymatically digested non-buoyant cellular fraction constitutes the stromal vascular fraction. It contains ASCs, vascular progenitor cells, pericytes, and endothelial cells. Although ASCs are of mesodermal origin, under specific conditions they can potentially differentiate into multiple lineages of adipogenic, osteogenic, chondrogenic, myogenic, cardiomyogenic, and neurogenic cells. Recent studies further show that ASCs can differentiate into tissues of ecto- and endodermal lineages such as neural cells, hepatocytes, pancreatic islet cells, endothelial cells, and epithelial cells. There is evidence showing that stem cells contribute to the restoration of tissue vascularization and organ function. For these reasons, adipose tissue is potentially clinically usable for cellular therapy, tissue engineering, and gene transfer applications in regenerative medicine [26]. A study by Rigotti et al. demonstrates that ASCs is therapeutically effective for the treatment of radiation-induced damage by replacing damaged tissue with reconstructed normal tissue [27]. The use of adipose-derived stem cells essentially is an advancement in the treatment of different types of facial disorders, including maxillofacial deformities [28, 29].
Conclusion
Fat grafting, first used to improve facial aesthetics, has in recent years progressed into more and complex reconstructive procedures [8, 19]. This technique should not be considered just as an ancillary treatment but an integral part of the methods of the modern reconstructive surgeon and also as a basic complementary reconstructive surgical procedure. FG is an excellent tool because it yields natural-looking results that stand the test of time. Modern tissue engineering and regenerative medicine are multidisciplinary sciences that are evolving in parallel with biotechnological advances. The uses of stem cells in tissue repair and tissue regeneration are impressive. Recent research on stem cells indicates that the use of adult stem cells may also be equally powerful in treating congenital and acquired disorders. Yet, the difficult availability and the correct processes for obtaining stem cells are still a challenge for surgeons and scientists pursuing regenerative medicine. Fat volume retention is still challenging to predict and it largely depends on a right balance between regeneration and resorption of adipose tissue, which depends on many factors including graft size, procedure techniques, and graft microenvironment. The mechanism underlying the survival of adipose tissue after FG is not entirely understood. However, many studies have investigated this issue and several well-supported theories have been put forward. A better understanding of the biology and scientific principles that regulate adipose tissue will provide a better insight into ideal methods capable of producing long-lasting and desirable results. Laboratory and clinical data indicate that the use of adult stem cells is a very promising field with enormous potential both for therapeutic applications in various disease conditions and for aesthetic applications. Recent research on ASCs would suggest that adult stem cells may prove to be an equally powerful tool in treating congenital and acquired disorders. However, the availability of, and the processes for, obtaining stem cells remain a challenge for both surgeon and scientist pursuing regenerative medicine. The future will be shaped by research, laboratory and education, from bench to bedside [29].
Acknowledgments
The Senior Author, Luigi Clauser, wishes to acknowledge Dr. Sydney R. Coleman, TriBeCa Plastic Surgery New York NY, for developing and systematizing fat grafting techniques in the early 90’s, in research and clinical applications, from bench to bedside. Since the early 90’s Dr. Coleman still remains guide and inspirer in the everyday clinical practice at the Institution of Maxillo-Facial Surgery, Istituto Stomatologico, Milano, Italy.
Author Contributions
Francesco Gallo, Luigi Clauser made a substantial contribution in the surgical work and to conception and design of the article, designed the clinical data acquisition and research at the Clinical Institution in Milano, Italy; Luigi Clauser, Antonio Lucchi performed surgical treatments and samples collection; Luigi Clauser, Carolina Sannino, Federica Riva prepared the manuscript with numerous insights and suggestions.
Availability of Data and Materials
Not Applicable.
Financial Support and Sponsorship
None.
Conflicts of Interest
None.
Ethical Approval and Consent to Participate
Not Applicable.
Consent for Publication
Written informed consent was obtained for all patient images.
Article Info
Article Type
Review ArticlePublication history
Received: Mon 03, Jun 2024Accepted: Mon 17, Jun 2024
Published: Wed 31, Jul 2024
Copyright
© 2023 Luigi Clauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2024.01.01
Author Info
Luigi Clauser Federica Riva Antonio Lucchi Carolina Sannino Francesco Gallo
Corresponding Author
Luigi ClauserDepartment of Maxillo-Facial Surgery (Director and Chief: Francesco Gallo, MD) Istituto Stomatologico Italiano, Via Pace, 21 20122 MILANO, Italy
Figures & Tables






References
1.
Coleman
SR (1997) Facial recontouring with lipostructure. Clin Plast
Surg 24: 347-367. [Crossref]
2.
Coleman SR (2001)
Structural fat grafts: the ideal filler? Clin Plast Surg 28: 111-119. [Crossref]
3.
Coleman SR, ed.
(2004) Structural Fat Grafting. Saint Louis, MO: Quality Medical Publishing
Inc.
4.
Rigotti G, Marchi
A, Sbarbati A (2009) Adipose-derived mesenchymal stem cells: past, present, and
future. Aesthetic Plast Surg 33: 271-273. [Crossref]
5.
Strem BM, Hicok KC,
Zhu M, Wulur I, Alfonso Z et al. (2005) Multipotential differentiation of
adipose tissue-derived stem cells. Keio J Med 54: 132-141. [Crossref]
6.
Moseley TA, Zhu M,
Hedrick MH (2006) Adipose-derived stem and progenitor cells as fillers in
plastic and reconstructive surgery. Plast Reconstr Surg 118: 121S-128S.
[Crossref]
7.
Clauser LC, Tieghi
R, Galiè M, Carinci F (2011) Structural fat grafting: facial volumetric
restoration in complex reconstructive surgery. J Craniofac Surg 22:
1695-1701. [Crossref]
8.
Giugliano C,
Benitez S, Wisnia P, Sorolla JP, Acosta S et al. (2009) Liposuction and
lipoinjection treatment for congenital and acquired lipodystrophies in
children. Plast Reconstr Surg 124: 134-143. [Crossref]
9.
Coleman SR,
Saboeiro A, Sengelmann R (2009) A comparison of lipoatrophy and aging: volume
deficits in the face. Aesthetic Plast Surg 33: 14-21. [Crossref]
10.
Bucky LP, Kanchwala
SK (2007) The role of autologous fat and alternative fillers in the aging face.
Plast Reconstr Surg 120: 89S-97S. [Crossref]
11.
Mojallal A,
Foyatier JL (2004) The effect of different factors on the survival of
transplanted adipocytes. Ann Chir Plast Esthet 49: 426-436. [Crossref]
12.
Pu LLQ, Coleman SR,
Cui X, Ferguson REH Jr, Vasconez HC (2008) Autologous fat grafts harvested and
refined by the Coleman technique: a comparative study. Plast Reconstr Surg
122: 932-937. [Crossref]
13.
Mojallal A,
Auxenfans C, Lequeux C, Braye F, Damour O (2008) Influence of negative pressure
when harvesting adipose tissue on cell yield of the stromal-vascular fraction. Biomed
Mater Eng 18: 193-197. [Crossref]
14.
Mojallal A, Shipkov
C, Braye F, Breton P, Foyatier JL (2009) Influence of the recipient site on the
outcomes of fat grafting in facial reconstructive surgery. Plast Reconstr
Surg 124: 471-483. [Crossref]
15.
Kawamoto HK (2009)
Fat injection and craniofacial surgery. In: Coleman SR, Mazzola RF, eds. Fat Injection From Filling to Regeneration. Saint Louis, MO: Quality
Medical Publishing Inc 447-474.
16.
Clauser L (2009)
Optimizing maxillofacial and craniofacial results. In: Coleman SR, Mazzola RF,
eds. Fat Injection From Filling to Regeneration. Saint Louis, MO: Quality
Medical Publishing Inc 475-500.
17.
Coleman SR (2020)
Long-term survival of fat transplants: controlled demonstrations. Aesthetic
Plast Surg 19: 421-425. [Crossref]
18.
Fagrell D, Eneström
S, Berggren A, Kniola B (1996) Fat cylinder transplantation: an experimental
comparative study of three different kinds of fat transplants. Plast
Reconstr Surg 98: 90-96. [Crossref]
19.
Clauser L, Polito
J, Mandrioli S, Tieghi R, Denes SA et al. (2008) Structural fat grafting in
complex reconstructive surgery. J Craniofac Surg 19: 187-191. [Crossref]
20.
Clauser L, Tieghi R
(2009) Optimizing maxillofacial and craniofacial results. Presented at:
American Society of Plastic Surgeons Annual Meeting; Seattle, WA.
21.
Clauser L, Tieghi
R, Consorti G (2010) Parry-Romberg syndrome: volumetric regeneration by
structural fat grafting technique. J Craniomaxillofac Surg 38: 605-609.
[Crossref]
22.
Consorti G, Tieghi
R, Clauser L (2012) Frontal linear scleroderma: long-term result in volumetric
restoration of the fronto-orbital area by structural fat grafting. J
Craniofac Surg 23: e263-e265. [Crossref]
23.
Foyatier JL,
Mojallal A, Voulliaume D, Comparin J-P. Clinical evaluation of structural fat
tissue graft (Lipostructure) in Cranio-Maxillofacial Trauma and Reconstruction
7.
24.
Gutowski KA, ASPS
Fat Graft Task Force (2009) Current applications and safety of autologous fat
grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg
124: 272-280. [Crossref]
25.
Lee DH, Yang HN,
Kim JC, Shyn KH (1996) Sudden unilateral visual loss and brain infarction after
autologous fat injection into nasolabial groove. Br J Ophthalmol 80:
1026-1027. [Crossref]
26. Zuk PA (2010) The adipose-derived stem cell: looking
back and looking ahead. Mol Biol Cell 21: 1783-1787. [Crossref]
27.
Rigotti G, Marchi A,
Galiè M, Baroni G, Benati D et al. (2007) Clinical treatment of radiotherapy tissue damage by lipoaspirate
transplant: a healing process mediated by adipose-derived adult stem cells. Plast
Reconstr Surg 119: 1409-1422. [Crossref]
28.
Kokai LE, Rubin JP,
Marra KG (2005) The potential of adipose-derived adult stem cells as a source
of neuronal progenitor cells. Plast Reconstr Surg 116: 1453-1460. [Crossref]
29.
Clauser L, Zavan B,
Galiè M, Vittorio LD, Gardin C et al. (2019) Autologous fat transfer for facial augmentation: surgery and
regeneration. J Craniofacial Surg 30: 682-685. [Crossref]
30.
Fraser JK, Wulur I,
Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem
cells for biotechnology. Trends Biotechnol 24: 150-154. [Crossref]
31.
Clauser L, Sarti E,
Dallera V, Galiè M (2004) Integrated reconstructive strategies for treating the
anophthalmic orbit. J Craniomaxillofac Surg 32: 279-290. [Crossref]
32. Glasgold RA, Glasgold MJ, Lam SM (2009) Complications following fat transfer. Oral Maxillofac Surg Clin North Am 21 :53-58, vi. [Crossref]
33. Caviggioli F, Klinger F, Villani F, Fossati C, Vinci V et al. (2008) Correction of cicatricial ectropion by autologous fat graft. Aesthetic Plast Surg 32: 555-557. [Crossref]
