Thoracic Endovascular Aortic Repair (TEVAR) for Primary Retrograde Type A Aortic Dissection
A B S T R A C T
Acute aortic dissection is an emergent and life-threatening condition. Retrograde Type A dissection (RTAD) can occur primarily or due to a complication of Type B dissection that often requires open repair, a highly morbid operation. While management of Type A and Type B dissections is clear, the literature is sparse regarding management of RTAD. We describe a case where the patient presented with a Type B dissection complicated by retrograde propagation, resulting in RTAD. We performed a Thoracic Endovascular Aortic Repair (TEVAR) as an alternative to standard open repair. The patient tolerated the procedure well and was discharged with resolution of the false lumen. Instead of treating a radiographic finding with a highly morbid operation, it is important to evaluate if an endovascular approach is a reasonable option to treat RTAD. Each case must be evaluated individually, but we believe that TEVAR may be an alternative intervention in specific cases.
Keywords
Endovascular, Type B dissection, TEVAR, retrograde Type A dissection
Introduction
Acute aortic dissection is defined as a tear in the innermost layer of the aortic wall. This results in high blood pressure flow, creating a true and false lumen, and is an emergent and life-threating medical condition [1]. The DeBakey classification, introduced in 1960s, commonly used classification of aortic dissection based on anatomic description. The Stanford classification system, introduced in 1970, is the most widely used classification for aortic dissections and based on whether the ascending aorta is affected. Type A involves the ascending aorta and may progress to involve the arch and thoracoabdominal aorta. Type B involves the descending thoracic or thoracoabdominal aorta distal to the left subclavian artery without involvement of the ascending aorta [1]. Open surgical repair is the standard of care for Type A dissections while management for Type B dissections involves resuscitation, initiation of anti-impulse therapy, and pain control. An important complication resulting from Type B dissection is Retrograde Type A dissection (RTAD), which is a dissection that originates distal to the ascending aorta resulting in retrograde flap progression within the ascending aorta [2]. While the literature in acute management of Type A and Type B is lucid, there is paucity of literature in management of RTAD. Additionally, given the complexity of RTAD, the Society of Vascular Surgery and Thoracic Surgery reclassified the scheme for aortic dissection and concluded that the distinction between Type A and Type B should be predicated on entry tear location alone [1].
The true incidence of RTAD is unknown, but it is reported to have an estimated overall incidence of 1%- 4% with mortality as high as 42% [2]. While it is a known major complication of thoracic aortic endovascular repair (TEVAR), primary RTAD is much rarer. Some of the proposed mechanisms for RTAD include unfavourable aortic dissection anatomy, natural progression of initial aortic dissection, and iatrogenic complications relating to devices used or the TEVAR procedure itself [3]. Given the high mortality rate, open surgical repair is considered the treatment of choice to avert this life-threatening condition.
Case Description
A 50-year-old male with history of hypertension, noncompliant with medications, presented to the Emergency Department with acute onset of chest pain radiating to the back. The patient was diaphoretic and appeared to be in acute distress. He was afebrile and non-tachycardic but was hypertensive to 198/120. His laboratory tests were significant for a leukocytosis of 12,000 and a creatinine of 1.6 mg/dL. The patient did not have any history of smoking and had no known history of aortic aneurysm or coronary artery disease.
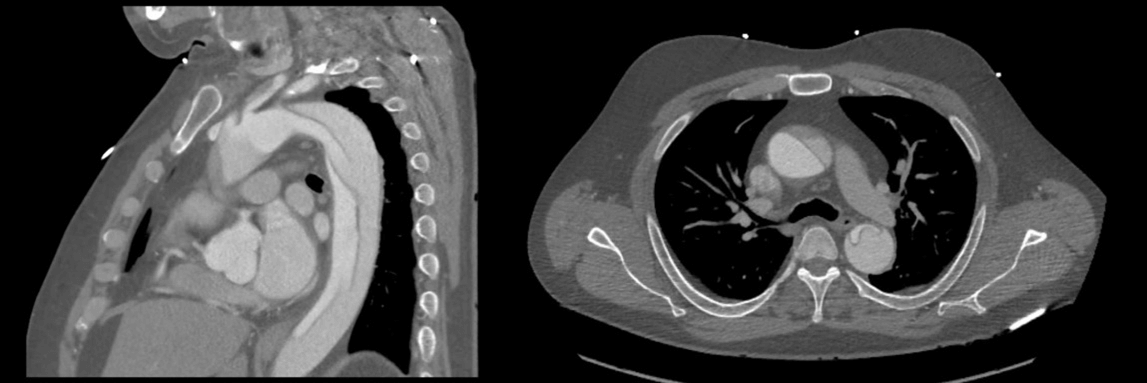
Given his severity and acuity of symptoms, the patient underwent CT angiography (CTA), which demonstrated an aortic dissection flap involving a portion of the ascending aorta and extending superiorly to the proximal portion of the left common carotid artery. The entry tear of the dissection was distal to the origin of the left subclavian artery and the false lumen extended inferiorly to the aortic bifurcation (Figure 1). Both true and false lumens were patent with no evidence of occlusion of any of the aortic branches. There was no evidence of pericardial effusion, aortic insufficiency, or coronary insufficiency on imaging. Treatment options were discussed including medical management, TEVAR, or a total arch replacement with endograft. Given that the retrograde Type A dissection originated distal to the left subclavian, we believed that TEVAR was the best approach for the case instead of a highly morbid open procedure. The patient elected to proceed with the endovascular approach.
Figure 1: Type A aortic dissection shown in CTA images. Sagittal view showing the entry tear in the descending aorta and distal to the subclavian artery and axial CTA view showing the false lumen extending into the ascending aorta.
The patient was taken to the operating room and the TEVAR was performed through ultrasound-guided cannulation of the right and left common femoral arteries. A pigtail was advanced through the left groin access into the ascending aorta with care to ensure that it was in the true lumen. Similarly, a Glidecath was advanced over a wire through the right groin sheath to the ascending aorta. A Double J Lunderquist wire was then advanced through the catheter and positioned in the ascending aorta. The initial thoracic angiogram performed demonstrated evidence of a dissection with false lumen filling from an origin in the descending thoracic aorta approximately 6 cm distal to the left subclavian artery origin. The angiogram did not clearly visualize an active false lumen flow in the thoracic arch or ascending aorta; however, there was flow seen on intra-operative transesophageal echocardiogram (TEE). A 36 × 161mm thoracic stent with proximal bare metal struts was advanced over the Lunderquist wire and deployed with the fabric immediately distal to the origin of the left subclavian artery. The pigtail was repositioned through the graft and in the ascending aorta. Angiogram demonstrated good proximal seal and location, with persistent early filling, and it was noted that the proximal/mid portion of the graft was not fully expanded. Angioplasty was performed to mold this portion of the stent graft with improved seal and expansion. Completion angiogram demonstrated residual filling of the false lumen without retrograde Type A dissection. TEE demonstrated improved apposition with no visualized flow into the false lumen of the ascending aorta.
Given that the patient was heparinized with known distal fenestrations, we suspected that coverage of the proximal entry tear would be adequate to decrease false lumen flow. In addition to continued blood pressure control, we predicted that we would be able to prevent further retrograde dissection. Arterial closure devices were deployed at the access sites with good haemostasis. We obtained a CTA on post-operative day three, which showed that the false lumen within the ascending aorta had thrombosed with expected false lumen filling in the descending thoracic aorta distal to the endograft. (Figure 2).
Figure 2: CTA images obtained on post-operative day 3 show graft extending from the level of the left subclavian artery into the descending thoracic aorta and show resolution of the false lumen extension into the ascending aorta.
Discussion
RTAD pathogenesis has been a point of contention in many studies. While some postulate that primary RTAD might be related to the natural progression of an underlying disease such as Type B aortic dissection, others report that RTAD is most commonly secondary to procedure related events, such as TEVAR, ballooning, or wire and sheath manipulation in the aortic arch. The fragility of the aortic wall has been considered a driving reason and primary mechanism of the pathogenesis of the disease. Mortality rates after RTAD have been reported up to 42%, including sudden deaths, and have shown to be higher than the rate for spontaneously occurring acute Type A aortic dissection [4]. Analysis has shown that patients in whom RTAD occurred as a result of TEVAR had worse outcomes compared with patients in whom RTAD occurred during follow-up [4]. In these situations, open surgical repair is often recommended once RTAD is diagnosed, with treatment options including replacement of the entire aortic arch with suturing of the vascular graft directly to the endograft. Other options include the complete removal of the endograft and Dacron graft replacement using the modified “elephant trunk” technique. Few cases of RTAD have been managed conservatively [2].
Although an open approach is the traditional method to treat a Type A dissection, there is documented evidence of potentially significant adverse complications. We elected the endovascular approach with its associated risks and benefits for our patient given the specific radiographic and echocardiographic appearance. We believe an endovascular approach in select cases of RTAD will be a better option than the open approach as it would allow for enhanced recovery after surgery.
Disclosures
None.
Informed Consent
Written informed consent was obtained by the patient, and no IRB was required since all patient information was removed and de-identified.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Tue 03, Jan 2023Accepted: Mon 23, Jan 2023
Published: Wed 15, Feb 2023
Copyright
© 2023 Syed F. Haider. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2023.01.01
Author Info
Syed F. Haider Joe T. Huang Shyamin Mehra Huzaifa Shakir
Corresponding Author
Syed F. HaiderDepartment of Surgery, Rutgers New Jersey Medical School, Newark, New Jersey, USA
Figures & Tables

References
1. Lombardi JV, Hughes
GC, Appoo JJ, Bavaria JE, Beck AW et al. (2020) Society for Vascular Surgery
(SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B
aortic dissections. J Vasc Surg 71: 723-747. [Crossref]
2. Estrera AL, Shah P,
Lee TY, Irani AD, Safi HJ (2009) Repair of retrograde type a aortic dissection
after thoracic endovascular aortic aneurysm repair using the modified elephant
trunk technique. Vascular 17: 116-120. [Crossref]
3. Kpodonu J, Preventza O, Ramaiah VG, Shennib H, Wheatly 3rd GH et al. (2008) Retrograde type A dissection after endovascular stenting of the descending thoracic aorta. Is the risk real? Eur J Cardiothorac Surg 33: 1014-1018. [Crossref]
4. Piffaretti G, Mariscalco G, Tozzi M, Bruno VD, Sala A et al. (2010) Acute iatrogenic type A aortic dissection following thoracic aortic endografting. J Vasc Surg 51: 993-999. [Crossref]
