Ulcerative Budding Skin Tumor Revealing Malignant Pleural Mesothelioma
A B S T R A C T
Pleural mesothelioma is a malignant tumor, which develops from the pleural mesothelial lining. We report a clinical observation of a 24-year-old young man who presented with a right serohematic pleurisy and ulcerative budding skin lesion of the right abdominal wall. Through this case, we highlight a very rare, atypical metastatic localization that can be clinically confused with a primary skin tumor.
Keywords
Mesothelioma, skin metastasis, ulcerative budding mass
Introduction
Mesothelioma is a malignant tumor, which develops from the mesothelial lining of the serosa, according to different growth patterns [1]. It occurs mainly at the pleural level (90% of cases), then at the peritoneal level (5 to 10% of cases), very rarely in the pericardium (0.4% of cases) and the testicular vagina [1, 2]. Classically, MMP preferentially affects men over the age of 60 who have been exposed to asbestos for at least 20 to 30 years. The link between exposure to asbestos and the development of an MMP was clearly established in the early 1960s [3]. Few studies of MMP in children and young adults have been performed. A review of the literature dating from 2010 made it possible to identify some data concerning mesothelioma affecting individuals under 20 years of age [4]. We report a clinical observation of a 24-year-old young man who presented with a right serohematic pleurisy and ulcerative budding skin lesion of the right abdominal wall. Through this case, we highlight a very rare, atypical metastatic localization of MMP, which can be clinically confused with a primary skin tumor.
Clinical Observation
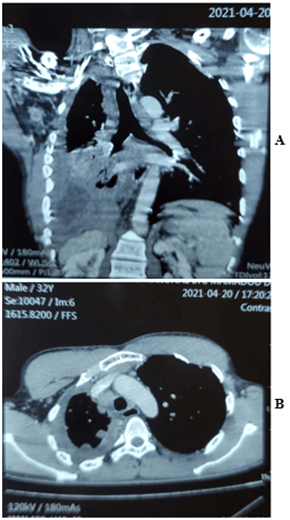
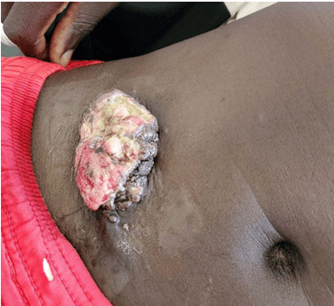
On May 21, 2021, we admitted to the pulmonology department a 24-year-old unmarried farmer without children for moderate dyspnea on the effort, which had progressed for 1 month before his admission in a context of chest pain and pronounced deterioration of the condition in general. This patient had no particular pathological history, apart from active smoking ten pack-years, weaned for 5 years. No notion of occupational exposure to asbestos was noted. The general examination had noted a patient with a poor general hyperalgesic state and very short of breath at the slightest effort. Pleuropulmonary examination showed right fluid effusion syndrome. Mucosal examination showed a recent ulcerative budding skin lesion of the anterior abdominal wall that began as a small, hard and painful nodule (Figure 2). The thoraco-abdominal CT scan revealed right pleurisy with thickening of the pleura and a mass of the abdominal wall at the level of the right iliac fossa based on subcutaneous implantation without peritoneal invasion (Figures 1A & 1B). Thoracentesis had confirmed pleurisy with the evacuation of non-coagulating, non-exudative serohaematic fluid. The bacteriology as well as the Xpert MTB Rif test of pleural fluid were negative. The blind pleural biopsy was not helpful.
Faced with this clinical picture, we suggested as a diagnosis a primary skin cancerous lesion with pleuropulmonary metastatic involvement. A biopsy of the skin lesion taken immediately afterwards was in favour of a secondary localization with evidence of cancer cells of mesothelial origin (Figure 3). We performed a pleuroscopy which revealed numerous whitish tumoral-like lesions on the visceral and parietal pleura. Histological samples at this level confirmed the presence of malignant epidermoid-type mesothelioma.
Figure 1: A) Reconstructed CT section showing a mass of the abdominal wall at the level of the right iliac fossa based on subcutaneous implantation without peritoneal invasion. B) CT scan of the chest showing right pleurisy with a scalloped pleura that is thickened.
Figure 2: Ulcerative budding skin tumor of the right abdominal wall.
Figure 3: Pathological anatomy report of the biopsy specimen of the skin lesion.
Discussion
The first studies which demonstrated a link between exposure to asbestos and the development of an MMP date back to the 1960s. More than 80% of MMPs in men have a history of exposure to asbestos [5]. The latency time between the first exposure and the development of mesothelioma is rarely less than 20 years, often of the order of 30 to 40 years, or even more [6]. Occupational exposure to asbestos is the main risk factor for mesothelioma. A history of asbestos exposure exists in almost all of the cases listed. However, mesothelioma has been reported in a few cases of individuals with no known exposure to asbestos. The pathogenesis of MMP in young adults and children, therefore, does not appear to be quite the same, with asbestos playing a less prominent role. Considering the age of our patient, who is 24 years old, and the lack of occupational exposure, the risk factor for this disease remains very difficult to establish. A few studies have associated the development of mesothelioma with infection of simian virus 40 (SV40) as well as the existence of certain genetic mutations [7-9]. In our African context, where the medical platform remains rudimentary, it is impossible to look for these genetic factors. The clinical signs are nonspecific. Chest pain, shortness of breath, fatigue and loss of appetite have been reported. In our observation, we found these same clinical signs with a duration of the disease of 1 year. Even if it can be detected by a simple chest X-ray, the reference examination is the chest CT [10]. In our patient, the thoraco-abdominal CT scan revealed pleurisy with scalloping of the visceral pleura, which thickened and a mass of the abdominal wall at the level of the right iliac fossa based on subcutaneous implantation without peritoneal invasion (Figure 1).
The diagnosis is made by pathological analysis of a sample of the tumor taken by pleural biopsy or by thoracoscopic or mediastinoscopic surgery [11]. In our patient, the diagnosis was made by biopsy under pleuroscopy and biopsy of the skin lesion. Three histological types have been described, including the epithelioid form, which represents more than half of the cases [12, 13]. In our patient, histological samples had confirmed the presence of malignant squamous-type mesothelioma.
Metastases distant from the tumor site are exceptional. They mainly sit on the cephalic extremity and usually occur when the diagnosis of primary cancer of the pleura has already been made. The majority of skin lesions are located near the tumor site and reflect either a direct extension of the tumor to the chest wall or by permeation nodule with a graft of carcinomatous cells by diffusion of pleural fluid at the time of diagnostic or therapeutic procedures. In our observation, distant metastatic involvement could be explained by diaphragmatic involvement with the invasion of cancerous ones to the subcutaneous level of the right iliac fossa.
Conclusion
While exposure to asbestos and distant metastatic damage from malignant pleural mesothelioma is well known, the occurrence of this tumor in children without exposure to the risk factor and secondary skin locations remains exceptional. We report a case, the originality of which lies in the way in which this tumor is revealed, which can be clinically confused with a primary skin tumor.
Article Info
Article Type
Case ReportPublication history
Received: Mon 20, Dec 2021Accepted: Wed 05, Jan 2022
Published: Wed 19, Jan 2022
Copyright
© 2023 Samba Niang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2021.03.03
Author Info
Samba Niang Fatimata Binetou Rassoul Mbaye Maimouna Fafa Cisse Justin Eca Khady Thiam El Hadji Mamadou Ndiaye Amadou Diop Dia Yacine Dia Kane Ansoumana Diatta Nafissatou Oumar Toure Badiane
Corresponding Author
Samba NiangService de Pneumologie, CHRU Saint-Louis, Sénégal
Figures & Tables

References
1.
Christelle Combaz-Lair (2015) Aspects cliniques et
histopathologiques du mésothéliome pleural malin chez l’enfant et l’adulte
jeune: à propos d’une série de 47 cas. Médecine humaine et pathologie
dumas-01120484.
2.
Gross-Goupil M, Ruffie P (1999) [Malignant pleural
mesothelioma]. Bull Cancer 3: 43-54. [Crossref]
3.
Wagner JC, Sleggs CA, Marchand P (1960) Diffuse pleural
mesothelioma and asbestos exposure in the North Western Cape Province. Br J
Ind Med 17: 260-271. [Crossref]
4.
Kashanskiy SV, Andre N (2010) [Paediatric mésothelioma: is
there the such a disease?]. Bull Cancer 97: 571-576. [Crossref]
5.
Galateau F, Brambilla E, Cagle P, Churg A, Colby T et al.
(2006) Pathology of Malignant Mesothelioma: Springer-Verlag London Limited
2006.
6.
Janes SM, Alrifai D, Fennell DA (2021) Perspectives on the
Treatment of Malignant Pleural Mesothelioma. N Engl J Med 385:
1207-1218. [Crossref]
7.
MacLachlan DS (2002) SV40 in human tumors: new documents shed
light on the apparent controversy. Anticancer Res 22: 3495-3499. [Crossref]
8.
Testa JR, Cheung M, Pei J, Below JE, Tan Y et al. (2011)
Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet
43: 1022-1102. [Crossref]
9.
Panou V, Røe OD (2020) Inherited Genetic Mutations and
Polymorphisms in Malignant Mesothelioma: A Comprehensive Review. Int J Mol
Sci 21: 4327-4327. [Crossref]
10. Woolhouse
I, Bishop L, Darlison L, Fonseka DD, Edey A et al. (2018) British Thoracic
Society guideline for the investigation and management of malignant pleural mesothelioma.
Thorax 73: i1-i30. [Crossref]
11. Bibby
AC, Tsim S, Kanellakis N, Ball H, Talbot DC et al. (2016) Malignant pleural
mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir
Rev 25: 472-486. [Crossref]
12. Blum Y, Meiller C, Quetel L, Elarouci N, Ayadi M et al. (2019) Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat Commun 10: 1333. [Crossref]
13. Wald O, Groth SS, Burt BM, Sugarbaker DJ (2016) Role of thoracoscopy, mediastinoscopy and laparoscopy in the diagnosis and staging of malignant pleural mesothelioma. J Vis Surg 2: 129. [Crossref]
