Urinary Cytology in the Diagnosis of Bladder Cancer
A B S T R A C T
The examination of urine is an ancient medical procedure dating back thousands of years. Microscopic examination of cells in the urine is being done since the invention of microscope. Presently the cytological examination of urine or other fluid samples from the urinary tract is a routine non-invasive diagnostic procedure to detect cancer of the urinary tract, especially in patients with painless haematuria. It is also used in the follow-up of patients previously treated for bladder cancer to detect recurrence or a new primary. It is a highly specific method for the diagnosis of invasive and in situ urothelial carcinoma and high-grade papillary carcinoma. However, it is unreliable for the detection of low-grade papillary neoplasms. Malignant cytomorphological characteristics of the exfoliated cells in urine or bladder washing can facilitate the diagnosis of bladder cancer. The Paris System (TPS) Working Group has proposed. The Paris System (TPS) authorities have proposed a standard reporting stem which includes specified diagnostic categories and cytomorphologic criteria for diagnosis of High-grade Urothelial Carcinoma (HGUC).
Keywords
Cytology, urine, bladder washing, urothelial carcinoma
Introduction
Dr. George Papanicolaou hypothesized more than five decades ago; the urinary tract malignancies can be detected by evaluation of exfoliated cells in urine [1]. Microscopic evaluation of stained cellular smears from the urine is termed as urinary cytology. Urinary cytology is not a laboratory test and needs a pathologist’s interpretation of the morphologic features of shed urothelial cells. However, urinary tract cytology has been plagued with poor sensitivity, accuracy, and reproducibility. Several factors such as the low sensitivity in detecting low-grade non-invasive lesions, lack of standardized diagnostic criteria, and wide inter-observer variability are particularly worrisome [2]. Urine cytology samples constitute a significant percentage of daily case volume in any cytopathology practice. Although the numbers of urinary cytology are significantly less when compared to the gynaecologic cases, it is a more difficult specimen that pathologists encounter. Problems encountered by cytopathologists include inadequate cellularity of samples, cellular degeneration prior to fixation in cytology for diagnosis of Low-grade urothelial neoplasms (LGUN). 70% of the bladder tumors encountered via cystoscopy are LGUN [1, 3].
It is well known that the Bethesda System (TBS) for reporting Cervical Cytology terminology, which was initiated in 1988, has led the way for standardized reporting in cytopathology [4]. However, there has been a lack of a standardized/comprehensive reporting system for urinary cytology, which is based on the current understanding of urothelial carcinoma (UC). An international panel of cytopathologists met in Paris in May 2013 on the occasion of the 18th International Congress of Cytology organized by the International Academy of Cytology, to set a standard reporting protocol of urinary cytology [1]. The value of ancillary tests in the screening and diagnosis of urinary neoplasms was also considered during this meet.
The Urologist’s Perspective
Urologists depend on routine imaging, endoscopic evaluation of the urinary tract, and urinary cytology in order to detect a potential malignancy. The majority of bladder cancers are low-grade and non-invasive and despite observing a well-defined papillary bladder tumor on direct cystoscopic examination, the urine cytology may be ‘negative’. The detection of low grade and non-invasive lesions by cytology has known limitations as there is a low risk of progression. In comparison, when it comes to high-grade urothelial neoplasms (HGUC), there seems to be more reliability of cytology to rule out the tumors in situ. These lesions have a potential for recurrence, invasion, metastases, and morbidity/mortality; therefore, patients with HGUC reflect the high-risk population and benefit from surveillance evaluation with non-invasive urine cytology.
In an appropriate clinical setting, urine cytology plays an important adjunctive role, moreover, the test is relatively cheap and collection methods are either minimally invasive or non-invasive. An initial evaluation in patients at higher risk for bladder cancer (older age, male, smoking history, occupational exposures) and those with unexplained irritative urinary symptoms (potentially due to carcinoma in situ) should include urine cytology. Some urologists also recommend the use of cytology in the initial diagnosis and surveillance for HGUC [5-7]. This can be performed at the time of cystoscopy, during which a bladder washing/barbotage can be easily acquired and it has a high cellularity. Urine cytology also represents important means to survey the upper tracts and urethra in case of radical cystectomy with urinary diversions. Urinary cytology has its limitations. Studying morphology under the microscope is not a perfect reflection of biologic behaviour. Disease-related factors (poor sensitivity for low-grade non-invasive tumors), the method of sampling (voided versus instrumented), and the experience of the cytopathologist may all be responsible. These limitations must be understood by the urologists when interpreting the reports. Communication between the urologists and cytopathologists is very important to improve the clinical utility of urine cytology.
Preparation of Fluid Samples for Cytopathological Examination
Spontaneously voided urine is the most common sample sent for cytopathological examination. Apart from that, bladder wash samples catheterized urine samples or urine obtained by retrograde catheterization of the ureters or renal pelvis are occasionally sent for the cytopathological examination. The most ideal sample which contains sufficient number of preserved cells is the second morning voided urine sample. The first morning or overnight urine contains more cells but shows different degrees of degeneration as they are exposed to the acid milieu of urine throughout the night and are less suitable for the cytological evaluation. As the urothelial cells exfoliate intermittently, it would be right to examine three urine samples from three consecutive days to ensure that diagnostic cells are adequately sampled [8].
The bladder wash sample is obtained during or before cystoscopy and is an invasive diagnostic procedure. First, the bladder is emptied using a catheter and then 50 to 100ml of normal saline is instilled and recovered for three times. The bladder cells get exfoliated into the washings and are used for microscopic examination. These bladder washing samples are highly cellular and contain well-preserved cells. In cases wherein the urinary sample cannot be delivered to the cytology laboratory within three hours after they have been obtained, then it needs to be prefixed with a mixture of 2% polyethylene glycol and 50% to 70% ethanol [9]. Several different techniques are used for the cytopathological preparation of the fluid samples. Centrifugation of fluid is used by some laboratories to obtain a pellet, which is then directly smeared onto the glass slide. While some laboratories use the commercially available ThinPrepTM technique for the preparation of samples from the urinary tract [9, 10]. The majority of erythrocytes and leukocytes are removed because the gentle negative pressure that is applied to assist filtration, usually deforms these cells and they pass through the filter. A single layer of cells (monolayer) is obtained by gently imprinting the filter onto a pair of glass slides. The cell sample on the slide is fixed by and the cell preparations are subsequently stained by the Papanicolaou method [9].
Diagnostic Categories and Morphologic Criteria
The goal of any standard diagnostic system should be to define the morphologic criteria for the various categories in urinary tract cytopathology and also to standardize the reporting system to be universally acceptable and globally utilized. The Paris System for Reporting Urinary Cytology has laid down the following criteria as shown in (Table 1) [1].
Table 1: Diagnostic categories.
|
1 |
Non-diagnostic/unsatisfactory |
|
2 |
Negative for high-grade urothelial
carcinoma (NHGUC) |
|
3 |
Atypical urothelial cells (AUC) |
|
4 |
Suspicious for high-grade urothelial
carcinoma (SHGUC) |
|
5 |
High-grade urothelial carcinoma (HGUC) |
|
6 |
Low-grade urothelial neoplasm (LGUN) |
|
7 |
Other: primary and secondary malignancies
and miscellaneous lesions |
I Adequacy
Adequacy of cells in a specimen is an integral part of the report. Adequacy in some specimen types has been clearly defined such as cervicovaginal cytology, fine needle aspiration (FNA) specimens of the thyroid and adequacy criteria have also been proposed for pancreaticobiliary system cytology [11-15]. Adequacy of the sample also ensures that the specimen is representative of what is sampled. If the sample does contain abnormal cells, the specimen is considered ‘adequate for diagnosis’, no matter how few. Sometimes the definition of adequacy is based on the quantification or at least a semi-quantification of the cell count and/or volume of voided urine. Recently an evidence-based study has suggested that 2,600 cells or 2 well-visualized urothelial cells per high-power field in 10 consecutive high-power fields can serve as objective measure of adequacy in urine using ThinPrep method. Barkan in another study evaluated the volume of voided urine, concluding that specimens larger than 30 ml are more likely to be cellular/satisfactory [16, 17].
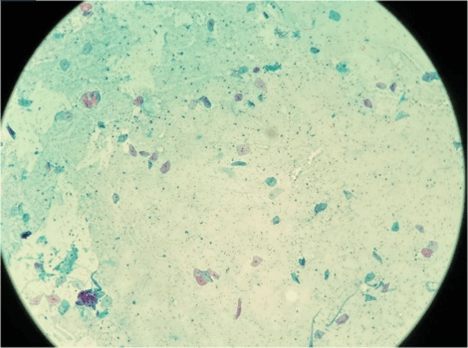
Figure 1: Shows normal urothelial cells (100 X), Papanicolaou staining.
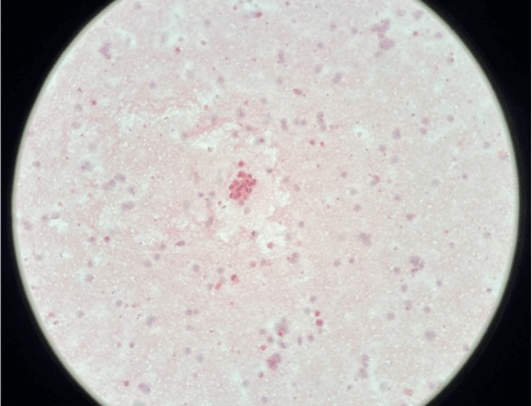
Regardless of the specimen type (voided urine or instrumented), if the urothelial cells are completely obscured by inflammatory cells or lubricants then it is termed as ‘unsatisfactory’. Contrarily, presence of atypical cells regardless of overall cellularity is termed as ‘Satisfactory’ (Figures 1 & 2).
Figure 2: A cluster of malignant urothelial cells having raised nucleocytoplasmic ratio (200 X).
II High-Grade Urothelial Carcinoma
The cytomorphologic characteristics of HGUC has remained unchanged since the days of George Papanicolaou and Leopold Koss [1]. HGUC has been well recognized, having the following features: high Nucleus/Cytoplasm (N/C) ratio, nuclear pleomorphism, nuclear membrane irregularity, and severe hyperchromasia [18, 19]. Also, malignant cells having eccentric nuclei with dense cytoplasm, coarse chromatin, high nucleo-cytoplasmic pleomorphism, are well described and illustrated. Other notable features like presence of mitotic figures and apoptotic bodies were also seen [1]. The Paris system reports that the necessary morphological features to diagnose HGUC should include: a minimum of 5 to 10 severely abnormal urothelial cells with an N/C ratio of ≥0.7 , with cells showing moderate to severe hyperchromasia, coarse chromatin, and markedly irregular nuclear membrane [1].
III Low-Grade Urothelial Neoplasm
The diagnosis of LGUN is based on features such as minimal nuclear enlargement, nuclear membrane irregularity, the density of cytoplasm, and elongated nuclei [20, 21]. The Paris system acknowledges that in the majority of cases a reliable diagnosis of low-grade carcinoma cannot be made, with the morphologic features listed above [1]. Presence of fibrovascular cores, a feature extremely rare in urine specimens, is the only instance when the diagnosis of a low-grade papillary lesion in instrumented urine can be made with confidence [1].
Ancillary Urine-Based Techniques for the Diagnosis of Urothelial Bladder Cancer
The urinary cytology examination of urine or bladder washing cell samples is very specific (97%) but has low sensitivity in case of low-grade papillary tumors [8, 9, 22-26]. Quite often the urologist faces a dilemma when the urine cytology report makes a diagnosis of ‘atypical urothelial cells’ which is inconclusive for malignancy, especially in patients with negative or equivocal findings on endoscopy. Several ancillary studies can be used along with urine cytology. A few of them are currently approved by the US Food and Drug Administration (FDA)- namely: UroVysion FISH (Abbott Molecular Inc, Des Plaines, Ill., USA), ImmunoCyt (Scimedx, Denville, N.J., USA), BTA stat (Polymedco, Cortlandt Manor, N.Y., USA), and NMP 22 (Allere, Waltham, Mass., USA). The FDA approval for these tests is for voided urine specimens only.
I DNA Ploidy
During the seventies and eighties of the last century, researchers and pathologists commonly used DNA cytometry to measure DNA ploidy of urothelial tumors [9, 27]. It was noted that the non-invasive low- grade urothelial tumors were mostly diploid, whereas Grade II urothelial carcinomas were diploid in 50% of the cases and aneuploid in rest 50%. However in case of Grade III tumors and carcinomas in situ were predominantly aneuploid. Aneuploid tumors when clinically correlated were associated with tumor persistence, recurrence, and progression to invasion [28].
II BTA Stat
Bladder Tumor Antigen Test detects the basal membrane antigen (complement factor H-related protein) in the urine using immunoassay (latex agglutination test) [24]. The test showed variable sensitivity (34%-100%) and especially its sensitivity for low-grade tumors was moderate and specificity was in the range 40-96%. But a high false positive rate of (4-34%) makes it debatable for clinical use.
III NMP22 (Nuclear Matrix Protein)TM Immunoassay
NMP22 is a member of the family of nuclear matrix proteins that are involved in DNA configuration, structure, and function [24]. The NMP22TM detection method is an immunoassay that showed high sensitivity (60-86%) for the detection of urothelial neoplasia, however the specificity is below that of cytology (48-81%) producing many false-positive tests. The FDA has approved this test to detect bladder cancer in voided urine, an adjunct to cystoscopy.
IV 5-Aminolevulinic Acid-Induced Fluorescent Urine Cytology
5-aminolevulinic acid (5-ALA) is a precursor in heme biosynthesis, and protoporphyrin IX, which is a metabolic product of 5-ALA, accumulates in the mitochondria following its administration [29]. It accumulates more in tumor cells than in healthy cells. Protoporphyrin IX emits red fluorescence when excited with a blue light at a wavelength of 405 nm, has a peak at a wavelength of 635 nm, and is used to visualize cancer cells (Figures 3A, 3B & 3C). The sensitivity of urine cytology using 5-ALA has improved to 82% [30].
Figure 3: A) Normal urothelial cells with black background and showing no frequency and intensity of peaks. B) Suspicious urothelial cells with black background and shows low frequency and intensity of peaks. C) Malignant urothelial cells with Intense red colour and black background and shows high intensity and frequency of peaks.
Multitarget Multicolour Fluorescence In Situ Hybridization (FISH) UroVysionTM Test
UroVysionTM FISH Test
This FISH assay was developed to improve the detection of invasive bladder carcinomas in voided urine. It is now FDA-approved for initial diagnosis and surveillance of patients with hematuria [25]. FISH identifies fluorescently labeled DNA probes that bind to intranuclear chromosomes. The sensitivity and specificity of this test varies from 8 to 100% and 29 to 100% in various literatures [26]. This variability in the reported performance of the test may be due to the prevalence of the disease in the population tested, the specimen type (voided urine versus instrumented specimens), and the cellularity of the urinary specimen used for FISH testing. In general, this ancillary test might be of potential use for clarifying atypia in urinary cytology and may be able to assist the urologist in clinical management. Yoder et al. has suggested that if the cytology is positive and used as the first diagnostic test then no UroVysionTM test was necessary, as cytology is nearly 100% specific [30]. However, if cytology was negative or atypical cells were found, then the reflex UroVysionTM test was performed on the same urine or bladder washing specimen. The atypia in urinary cytology may be clarified using the ancillary tests but it has to be performed in the hands of an experienced cyto-morphologist, under consideration of cystoscopy findings, and the patient’s medical history.
Conclusion
The main objective of urine cytology is to diagnose high-grade urothelial carcinoma (HGUC). It is very important for the urologist or the treating Physician to know the limitations of urinary cytology and to utilize urine cytology and non-invasive ancillary tests thoughtfully and practically to make the diagnosis.
Funding
None.
Conflicts of Interest
None.
Permissions
None.
Article Info
Article Type
Review ArticlePublication history
Received: Sat 19, Sep 2020Accepted: Tue 06, Oct 2020
Published: Fri 26, Mar 2021
Copyright
© 2023 R. B. Nerli . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2020.01.05
Author Info
R. B. Nerli Shadab Rangrez Saziya Bidi Shridhar C. Ghagane Shreya Chandra
Corresponding Author
R. B. NerliDepartment of Urology, JN Medical College, KLE Academy of Higher Education & Research (Deemed-to-be-University), JNMC Campus, Belagavi, Karnataka, India
Figures & Tables
Table 1: Diagnostic categories.
|
1 |
Non-diagnostic/unsatisfactory |
|
2 |
Negative for high-grade urothelial
carcinoma (NHGUC) |
|
3 |
Atypical urothelial cells (AUC) |
|
4 |
Suspicious for high-grade urothelial
carcinoma (SHGUC) |
|
5 |
High-grade urothelial carcinoma (HGUC) |
|
6 |
Low-grade urothelial neoplasm (LGUN) |
|
7 |
Other: primary and secondary malignancies
and miscellaneous lesions |

References
1. Barkan
GA, Wojcik EM, Nayar R, Savic Prince S, Quek ML et al. (2016) The Paris system
for reporting urinary cytology: the quest to develop a standardized
terminology. Acta Cytol 60: 185-197. [Crossref]
2. Blick
CGT, Nazir SA, Mallett S, Turney BW, Onwu NN et al. (2012) Evaluation of
diagnostic strategies for bladder cancer using computed tomography (CT)
urography, flexible cystoscopy and voided urine cytology: results for 778
patients from a hospital haematuria clinic. BJU Int 110: 84-94. [Crossref]
3. van
Rhijn BWG (2012) Combining molecular and pathologic data to prognosticate
non-muscle-invasive bladder cancer. Urol Oncol 30: 518-523. [Crossref]
4. Soloman
D (1989) The 1988 Bethesda system for reporting cervical/vaginal cytologic
diagnoses: Developed and approved at the national cancer institute workshop in
Bethesda, MD, December 12-13, 1988. Diagn Cytopathol 5: 331-334. [Crossref]
5. Babjuk
M, Burger M, Zigeuner R, Shariat SF, Van Rhijn BWG et al. (2013) EAU guidelines
on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur
Urol 64: 639-653. [Crossref]
6. Clark
PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE et al. (2013) Bladder
cancer. J Nat Compr Canc Netw 11: 446-475. [Crossref]
7. Koss
LG, Melamed MR (2006) The lower urinary tract in the absence of cancer. Koss’s
diagnostic cytology and its histopathologic bases. 5th edition. Philadelphia:
Lippincott Williams & Wilkins 738-776.
8. Flezar
MS (2010) Urine and bladder washing cytology for detection of urothelial carcinoma:
standard test with new possibilities. Radiol Oncol 44: 207-214. [Crossref]
9.
The ThinPrep PAP Test (2020).
10. Studeman
KD, Ioffe OB, Puszkiewicz J, Sauvegeot J, Henry MR (2003) Effect of cellularity
on the sensitivity of detecting squamous lesions in liquid-based cervical
cytology. Acta Cytol 47: 605-610. [Crossref]
11. Solomon
D, Nayar R (2004) The Bethesda System for reporting cervical cytology:
definitions, criteria, and explanatory notes. Springer Verlag.
12. Grant
CS, Hay ID, Gough IR, McCarthy PM, Goellner JR (1989) Long-term follow-up of
patients with benign thyroid fine-needle aspiration cytologic diagnoses. Surgery
106: 980-985. [Crossref]
13. Cibas
ES, Ali SZ (2009) The Bethesda system for reporting thyroid cytopathology. Thyroid
19: 1159-1165. [Crossref]
14. Pitman
MB, Centeno BA, Ali SZ, Genevay M, Stelow E et al. (2014) Standardized
terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou
Society of Cytopathology guidelines. Diagn Cytopathol 42: 338-350. [Crossref]
15. Prather
J, Arville B, Chatt G, Pambuccian SE, Wojcik EM et al. (2015) Evidence-based
adequacy criteria for urinary bladder barbotage cytology. J Am Soc
Cytopathol 4: 57-62. [Crossref]
16. Barkan
GA (2016) Enough is enough: adequacy of voided urine cytology. Cancer
Cytopathol 124: 163-166. [Crossref]
17. Koss
LG, Melamed MR (2006) Koss' diagnostic cytology and its histopathologic bases. Lippincott
Williams & Wilkins.
18. Papanicolaou
GN, Marshall VF (1945) Urine sediment smears as a diagnostic procedure in
cancers of the urinary tract. Science 101: 519-520. [Crossref]
19. Xin
W, Raab SS, Michael CW (2003) Low‐grade urothelial carcinoma: Reappraisal of
the cytologic criteria on ThinPrep®. Diagn Cytopathol 29: 125-129. [Crossref]
20. Murphy
WM, Soloway MS, Jukkola AF, Crabtree WN, Ford KS (1984) Urinary cytology and
bladder cancer. The cellular features of transitional cell neoplasms. Cancer
53: 1555-1565. [Crossref]
21. Bastacky
S, Ibrahim S, Wilczynski SP, Murphy WM (1999) The accuracy of urinary cytology
in daily practice. Cancer 87: 118-128. [Crossref]
22. Curry
JL, Wojcik EM (2002) The effects of the current World Health
Organization/International Society of Urologic Pathologists bladder neoplasm
classification system on urine cytology results. Cancer 96: 140-145. [Crossref]
23. Ross
JS, Cohen MB (2000) Ancillary methods for the detection of recurrent urothelial
neoplasia. Cancer Cytopathol 90: 75-86.
24. Sokolova
IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG et al. (2000) The
development of a multitarget, multicolor fluorescence in situ hybridization
assay for the detection of urothelial carcinoma in urine. J Mol Diagn 2:
116-123. [Crossref]
25. Dimashkieh
H, Wolff DJ, Smith TM, Houser PM, Nietert PJ et al. (2013) Evaluation of
urovysion and cytology for bladder cancer detection: a study of 1835 paired
urine samples with clinical and histologic correlation. Cancer Cytopathol 121:
591-597. [Crossref]
26. Böcking
A, Striepecke E, Auer H, Füzesi L (1994) Static DNA cytometry. Biological
background, technique and diagnostic interpretation. Wied GL, Bartels PH,
Rosenthal D, Schenk U (1994) Compendium on computerized cytology and histology
laboratory. Chicago: Tutorials of Cytology 107-128.
27. Tribukait
B (1987) Flow cytometry in assessing the clinical aggressiveness of
genito-urinary neoplasms. World J Urol 5: 108-122.
28. Malik
Z, Lugaci H (1987) Destruction of erythroleukaemic cells by photoactivation of
endogenous porphyrins. Br J Cancer 56: 589-595. [Crossref]
29. Nakai
Y, Anai S, Onishi S, Masaomi K, Tatsumi Y et al. (2015) Protoporphyrin IX
induced by 5-aminolevulinic acid in bladder cancer cells in voided urine can be
extracorporeally quantified using a spectrophotometer. Photodiagnosis
Photodyn Ther 12: 282-288. [Crossref]
30. Yoder
BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC et al. (2007) Reflex
UroVysion testing of bladder cancer surveillance patients with equivocal or
negative urine cytology: a prospective study with focus on the natural history
of anticipatory positive findings. Am J Clin Pathol 127: 295-301. [Crossref]
