Use of Accessible Blood Filter for Post-operative Cell Salvage in Cardiac Surgery
A B S T R A C T
The availability of centrifugal cell savers supports intraoperative cell salvage and thereby reduces the need for allogeneic red blood cell transfusion. Use of these devices, however, is limited to the operating room, forcing a switch to allogeneic products in the post-operative setting. Here we present a case of massive post-operative bleeding due to severe coagulopathy following CABG. Due to the lack of availability of donor blood products a novel blood filter (HemoClear BV, Zwolle, the Netherlands) was used for post-operative salvage. Because of its accessible use, we believe this salvage device has great clinical value in the poor-resource setting.
Keywords
Cardiac surgery, post-operative bleeding, cell salvage technology
Introduction
Bleeding during cardiac surgery is a major complication that often leads to anaemia and blood transfusion requirement [1]. In spite of the publication of patient blood management guidelines by various professional societies, implementation of blood conservation strategies remains challenging [2, 3]. As in the current COVID19 pandemic allogeneic blood shortages keep growing at an alarming rate, autologous blood transfusions are a very necessary relief of pressure on the donor blood supply system. The availability of centrifugal cell savers widely supports intraoperative cell salvage and thereby reduces the need for allogeneic red blood cell transfusion [4]. Use of these cell salvage devices is limited to the operating room, forcing a switch to allogeneic product in the post-operative setting. Moreover, mere salvage of red blood cells and loss of platelets and coagulation factors has been shown to contribute to coagulopathies [5, 6]. Here we present a case of massive post-operative bleeding due to severe coagulopathy following CABG. Due to the lack of availability of donor blood products and centrifugal cell saver outside of the operating room, a novel blood filter (HemoClear BV, Zwolle, the Netherlands) was used to salvage post-operatively shed blood cells [7].
Case Report
A male patient, aged 53 years, underwent an emergent surgical revascularization of the myocardium for a previously verified triple vessel coronary artery disease (CAD) with the LMCA at 90%, RCX 90%, RCA 90% stenose. The patient had a disease history of diabetes mellitus type 2, arterial hypertension, myocardial infarction, hyperlipidaemia and a positive family history of cardiovascular diseases.
Prior to the operation the patient’s haemoglobin level was 7.4 mmol/L, thrombocytes 321×109/L. The operation was completed without any complications. Upon leaving the operation room the patient had a haemoglobin level of 5.7 mmol/L and thrombocytes 285×109/L. The early post-operative recovery was stable for approximately two hours after which the patient gradually started to bleed more. In the third hour a blood loss of 120mL was measured. Despite normal coagulation parameters and hemodynamically stability, two FFPs were administered as well as tranexamic acid, desmopressin, and factor concentrates. Red blood cells and thrombocytes were not available at that moment because of large shortages caused by the COVID pandemic. During the following three hours, the blood loss became progressively more, and the patient became hemodynamically unstable. At this point in time, the haemoglobin level had fallen to 4.8 mmol/L and thrombocytes to 248×109/L. The patient was returned to the operating room to relieve the tamponade. The chest film and the ECG were unchanged. Extensive inspection showed no obvious cause for bleeding. Meanwhile, two RBC units had become available and were administered in addition to two FFPs, two units of TC and 550ml of salvaged blood cells from the cell saver. After which haemoglobin and thrombocytes were measured at 4.7 mmol/L and 153×109/L, respectively.
Perioperative TOE showed no special features. On departure from operating room the patient received high doses of vasopressor and inotropic agents. On the first day after surgery, after re-exploration the bleeding started again and within four hours there was a blood loss of 950mL. The haemoglobin level was now 3.1 mmol/L and thrombocytes decreased to 106×109/L. With the use of HemoClear filter (HemoClear BV, Zwolle, The Netherlands) we were able to wash the blood from the tubes and re-infuse 600ml of the patient’s own washed blood cells, with another two units of FFP. Following the autologous transfusion haemoglobin level was increased to 3.9 mmol/L, while the platelet count remained stable at 104×109/L. Over the following two to three hours dopamine and norepinephrine doses were decreased, the blood loss lessened, and the patient was successfully extubated on the first day post-operation. On the second day post-surgery the patient was transferred to the ward with a haemoglobin level of 4.1 mmol/L and thrombocyte count of 103×109/L. On day three post-surgery the patient received a unit of RBCs and started mobilizing with physiotherapist. The patient’s haemoglobin rose to 4.6 mmol/L on post-operation day four, the thrombocyte count was 170×109/L at this time. On post-operation day nine, the platelet count had further increased to 278×109/L, while the haemoglobin level remained at 4.5 mmol/L.
Conclusion
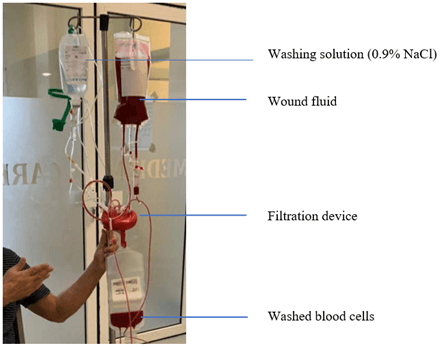
The novel HemoClear device enables accessible cell salvage and is easy to operate and affordable (Figure 1). Because the device is driven by gravity, it can be used in poor-resource settings without stable power supply. Particularly in emergent cases of nadir, the device could be a gamechanger in patient blood management.
Figure 1: The HemoClear post-operative salvage setup. By dilution with isotonic saline in the salvage system shed blood cells are washed and/or concentrated by the filter.
Acknowledgement
We would like to thank Dr. Arno Nierich (Cardiac Anaesthesiologist & Intensivist, Isala Hospital, The Netherlands) for his support in training on use of the blood filter and contributions to the cell salvage protocol. Financial support was provided from institutional and/or departmental resources. This use and research of this new technology was performed with full freedom of investigation.
Disclosure
None.
Article Info
Article Type
Case ReportPublication history
Received: Fri 20, Aug 2021Accepted: Sat 04, Sep 2021
Published: Fri 24, Sep 2021
Copyright
© 2023 Rosita Bihariesingh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJSCR.2021.02.01
Author Info
Rosita Bihariesingh Pieter Voigt Rakesh Bansie
Corresponding Author
Rosita BihariesinghDepartment of Anesthesiology & Intensive Care, Academic Hospital, Paramaribo, Suriname
Figures & Tables
References
1.
Colson PH, Gaudard P, Fellahi JL, Bertet H, Faucanie M et al.
(2016) Active Bleeding after Cardiac Surgery: A Prospective Observational
Multicenter Study. PLoS One 11: e0162396. [Crossref]
2.
Society of Thoracic Surgeons Blood Conservation Guideline
Task Force, Ferraris VA, Brown JR, Despotis GJ, Hammon JW et al. (2011) 2011
update to the Society of Thoracic Surgeons and the Society of Cardiovascular
Anesthesiologists blood conservation clinical practice guidelines. Ann
Thorac Surg 91: 944-982. [Crossref]
3.
Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G,
Surgical and Clinical Outcome Research (SCORE) Group (2013) Major bleeding,
transfusions, and anemia: The deadly triad of cardiac surgery. Ann Thorac
Surg 96: 478-485. [Crossref]
4.
Dalrymple Hay MJ, Dawkins S, Pack L, Deakin CD, Sheppard S et
al. (2001) Autotransfusion decreases blood usage following cardiac surgery -- a
prospective randomized trial. Cardiovasc Surg 9: 184-187. [Crossref]
5.
Murphy GJ, Allen SM, Unsworth White J, Terence Lewis C,
Dalrymple Hay MJR (2004) Safety and efficacy of perioperative cell salvage and
autotransfusion after coronary artery bypass grafting: a randomized trial. Ann
Thorac Surg 77: 1553-1559. [Crossref]
6. Paparella D, Whitlock R (2016) Safety of Salvaged Blood and Risk of Coagulopathy in Cardiac Surgery. Semin Thromb Hemost 42: 166-171. [Crossref]
7. Hoetink A, Scherphof SF, Mooi FJ, Westers P, van Dijk J et al. (2020) An In Vitro Pilot Study Comparing the Novel HemoClear Gravity-Driven Microfiltration Cell Salvage System with the Conventional Centrifugal XTRA™ Autotransfusion Device. Anesthesiol Res Pract 2020: 9584186. [Crossref]
