Use of Topical Nitroglycerine in the Neonatal Population Following Peripheral Tissue Ischaemia: Case Report and Updates about Safety Concerns
A B S T R A C T
Neonatal thrombosis is a well-described morbidity occurring in the neonatal intensive care unit. Critically ill neonates are most vulnerable to developing thrombosis with serious complications. Fingers and toes ischaemia secondary to vascular occlusion following central lines insertion remains an uncommon occurrence. The therapeutic approach for peripheral tissue injury using local warming, anticoagulants, thrombolytics or topical hyaluronidase showed limited benefits and potential side effects. Here we report an unusual case of finger ischaemia treated successfully using topical nitroglycerine. This complements previous reports confirming the efficacy and safety of this drug and highlighting its potential benefit in neonates.
Keywords
Neonatal thrombosis, nitroglycerine, limb ischaemia
Introduction
Thrombosis is an uncommon occurrence associated with higher morbidity in neonates; its incidence has been increasing in the past decade with similar involvement of the venous and arterial systems [1]. Newborns have an increased risk of thrombosis due to their decreased ability to inhibit thrombin and relative deficiency of fibrinolysis [2]. Other factors may lead to neonatal thrombosis, including congenital deficiencies of factor V Leiden, antithrombin, protein C and S, or elevated lipoprotein; in addition to acquired conditions like dehydration, polycythemia, septicemia and the presence of indwelling catheter [3]. Vascular thrombosis and right atrial thrombus are well-known complications of the umbilical venous catheter (UVC). However, ischaemia of the fingers or toes is exceptionally reported [4]. Treatment in those cases includes catheter removal, heat application to the contralateral site to promote vasodilation, and pharmacological agents such as anticoagulants, thrombolytics or topical use of hyaluronidase with limited benefits and potential side effects [5]. Here, we report on the successful treatment with topical nitroglycerine in a premature infant with finger ischaemia and we present a review on the modality and safety of such treatment.
Case Report
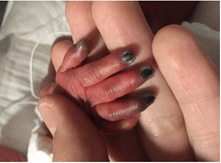
This was an 1115 grams extremely preterm female infant, member of twins product of 28 weeks and 2 days gestation, born to a 27-year-old, gravida 1, para 2 mother known to be homozygous for factor V Leiden deficiency. The pregnancy was complicated by preterm labour; the infant was delivered by caesarian section due to mal presentation. Apgar scores were 4, 6 and 8 at 1, 5 and 10 minutes, respectively. She was intubated and received one dose of surfactant for respiratory distress syndrome. The umbilical arterial line inserted at day one was removed on day 5 and the venous line on day 7 after birth. Twelve hours after removal of the former, the right thumb, index, middle, and ring fingers were noted to be cold and cyanosed (Figure 1). Hand elevation with warm compresses to the contralateral limb was applied. Four hours later, ischaemic changes became more evident with signs of early necrosis of the distal part of the fingers (Figure 2). The international normalized ratio (INR) and partial thromboplastin time (PTT) were normal. A venous duplex scan of the upper extremities and abdominal aorta showed normal blood flow with no evidence of thrombus. Arterial duplex scan of right radial and ulnar arteries could not be visualized due to technical difficulties. However, the handheld Doppler revealed a faint audible signal.
Figure 1: Initial presentation.
Figure 2: Progression of ischaemia.
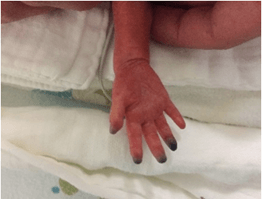
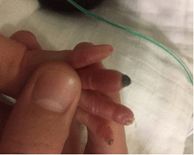
No prior attempts to place a radial arterial line nor to draw blood from that site were noted. A complete thrombophilia screen (antithrombin III, lupus anticoagulant, protein C, protein S and factors II, X, VII and MTHFR and factor V Leiden) was negative except for heterozygous state for factor V Leiden. On day 6 of life, Enoxaparin was started because worsening limb ischaemia progressed (Figure 2). Enoxaparin was discontinued 13 days later after the baby developed intraventricular hemorrhage (IVH) grade 1. As an alternative, a ribbon of 2% nitroglycerine ointment (4 mm/kg) was applied to the fingers, approximately 2 cm proximal to the line of pallor, every 8 hours. Improvement in color and perfusion was noted after the first dose. Methaemoglobin levels remained below 1%. By day 12 of treatment, the ischaemic area was limited to the fingertips only (Figure 3). Topical nitroglycerine was discontinued after 30 days when the fingers had recovered full perfusion and the nail beds were intact except for the ring finger, where half of the nail bed was lost (Figure 4). The baby was eventually discharged home at 37 weeks and 3 days postmenstrual age. She is currently healthy and has a minor loss of the right ring fingertip.
Figure 3: Day 7 of treatment.
Figure 4: Day 25 of treatment.
Discussion
Placement of central and peripheral catheters is a common procedure in the neonatal intensive care unit. However, ischaemic complications have been reported, especially in preterm critically ill patients [6]. In our case, the limb ischaemia, occurred distal from the line insertion, which is very unusual. One explanation would be a migrating thrombus from the umbilical arterial line. This is important to notice since acute limb ischaemia in neonates is a potentially life-threatening event that could lead to the death of nerves and muscle tissues within 4 to 6 hours unless the limb is re-vascularized, especially that neonatal blood vessels are more susceptible to rupturing, vasospasm and thrombosis, which increases the risk of ischaemia and necrosis in adjacent tissues [7, 8]. The initial treatment of this complication includes removal of the catheter, the elevation of the affected limb and application of heat to the contralateral region to promote vasodilation [5]. Occasionally, these measures are insufficient, and patients may require pharmacological treatment in the presence of a thrombus [9]. There are no well-established guidelines for the treatment of thrombotic events in neonates and individual decision-making remains necessary [10]. A majority of clinicians might decide to treat thrombosis with heparin in the absence of contraindications. Yet, research has shown that anticoagulants, thrombolytic and local infiltration of phentolamine or hyaluronidase offer limited benefits and cause side effects when administered systemically [9, 11].
The use of nitroglycerine as a salvage therapy for peripheral tissue injuries in neonates has been described since 1988 [12]. Nitroglycerine became commercially available as a 2% ointment in 1974 and has been used as a standard treatment for angina in adults. The initial case report of nitroglycerine use in a neonate was in 1988, where the patient with two sites of ischaemia caused by dopamine extravasation was treated with 2% topical nitroglycerine ointment instead of local infiltration of phentolamine; the ischaemia totally reversed [10]. More recently, 0.2% topical nitroglycerine ointment was used to treat ischaemia associated with umbilical and radial arterial lines at a lower dose (4 mm/kg) with apparent success and no observed side effects [13]. Although the available evidence is limited to case reports, all reports were consistent in the subjective or objective improvement of the affected limb’s perfusion following nitroglycerine therapy. In most cases, topical nitroglycerine was used as an adjuvant to physical measures [6]. Evidence of the efficacy of nitroglycerine in treating ischaemic complications associated with vascular cannulation or drug extravasation is limited to case reports in which all cases of limb ischaemia were near the site of cannulation, which is different from our case where limb ischaemia was not associated with peripheral arterial cannulation or pricking [5]. Nitroglycerine readily absorbed through intact skin forms a free radical nitric oxide, which activates guanylate cyclase and increases cellular guanosine 3’, 5’-cyclicmonophosphate. Nitroglycerine can increase collateral circulation to areas of peripheral ischaemia by cross-bridge dephosphorylating and relaxation of vascular smooth muscle [6, 12]. Due to the scarcity of studies and the lack of consensus on the safety and dosage of topical nitroglycerine, the use of this drug has been limited for fear of side effects, especially in preterm newborns, who have immature skin and a limited autoregulation of blood flow and therefore are at increased risk of a brain hemorrhage.
Studies in the literature have reported the use of a variety of doses in newborns ranging between 0.12 mg/kg and 2.5 mg/kg. Recent publications have established a dose of 1.22 mg/kg (4 mm/kg) as safe and efficacious [11]. The amount of absorbed nitroglycerine and systemic levels achieved is likely directly related to the quantity of ointment and size of the area of application [12]. The usual starting dose of 2% nitroglycerine ointment is 4 mm/kg (or 2 mg measured as 0.1mL in a syringe), which is equivalent to 0.2-0.5 𝜇g/kg/min administered intravenously [5]. The vasodilation is evident within 15-30 minutes, and the hemodynamic effects may be sustained for as long as six hours [12]. Preterm infants with immature epidermal permeability barriers can experience systemic complications because of the enhanced drug absorption [5]. This depends on the amount of nitroglycerine reaching the systemic circulation that varies directly with the size of the affected area and the amount of ointment applied. Hypotension and tachycardia were documented in a few reports but resolved in the majority of cases without treatment. Prolonged use of nitroglycerine may cause methaemoglobinemia therefore, monitoring of levels is important. However, this complication has only been noted in adults receiving high doses of intravenous nitroglycerine, about 1 mg/kg, which resulted in clinically significant (>10%) methaemoglobinemia [6, 12]. This report adds to the existing literature highlighting the possible occurrence of thrombotic occlusion distal from the line insertion and the possible complete reversal of injury using 2% nitroglycerine ointment in a preterm newborn without any adverse events. This complements the previous reports adding more knowledge about the efficacy and safety of this drug.
Conclusion
Topical nitroglycerine 2% ointment at a dose of 4 mm/kg may be a useful initial therapy for the reversal of tissue ischaemia in premature and term infants who do not respond to standard measures or in whom anticoagulation may increase the risk of bleeding. Although the available data is limited to case reports and case series, the optimum time for initiating nitroglycerine treatment after the ischaemic event, the dose and the frequency of administration remain to be determined. Further investigation with a randomized controlled trial on its use as solo therapy or in combination with anticoagulation is warranted.
Consent
Parental written permission to use photographs for education purposes is secured and will be provided if needed as supplementary information.
Data Availability
Not Applicable.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Tue 31, Aug 2021Accepted: Tue 14, Sep 2021
Published: Wed 29, Sep 2021
Copyright
© 2023 Lama Charafeddine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2021.02.02
Author Info
Mariam Anka Therese Saad Christelle Tayeh Lama Charafeddine
Corresponding Author
Lama CharafeddineDepartment of Pediatrics and Adolescent Medicine, Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Figures & Tables

References
1.
Haley KM (2017)
Neonatal Venous Thromboembolism. Front Pediatr 5: 136. [Crossref]
2.
Nosan G, Parom
Panjan D, Groselj Grenc M (2012) Thrombosis in newborns: experience from 31
cases. J Int Car Emer Medi 7: 29-32.
3.
Parasuraman S,
Goldhaber SZ (2006) Venous thromboembolism in children. Circulation 113:
e12-e16. [Crossref]
4.
Al Abdi SY, Dabelah
K, Mousa T, Al Algirim H (2015) Umbilical venous catheter-related ischemia of
fingers, right atrial thrombus treated with recombinant tissue plasminogen
activator and lethal pulmonary hypertension in a preterm infant. J Clin Neo
4: 199-202.
5.
Samiee Zafarghandy
S, van den Anker JN, Ben Fadel N (2014) Topical nitroglycerin in neonates with
tissue injury: A case report and review of the literature. Paediatr Child
Health 19: 9-12. [Crossref]
6.
Mosalli R, Elbaz M,
Paes B (2013) Topical Nitroglycerine for Neonatal Arterial Associated
Peripheral Ischemia following Cannulation: A Case Report and Comprehensive
Literature Review. Case Rep Pediatr 2013: 608516. [Crossref]
7.
Kayssi A, Shaikh F,
Roche Nagle G, Brandao LR, Williams SA et al. (2014) Management of acute limb
ischemia in the pediatric population. J Vasc Surg 60: 106-110. [Crossref]
8.
Arshad A, McCarthy
M (2009) Management of limb ischaemia in the neonate and infant. Eur J Vasc
Endovasc Surg 38: 61-65. [Crossref]
9.
Veldman A, Nold MF,
Michel Behnke I (2008) Thrombosis in the critically ill neonate: incidence,
diagnosis, and management. Vasc Health Risk Manag 4: 1337-1348. [Crossref]
10.
Denkler KA, Cohen
BE (1989) Reversal of dopamine extravasation injury with topical nitroglycerin
ointment. Plast Reconstr Surg 84: 811-813. [Crossref]
11.
Del Hoyo PV, Ruiz
PS, Del Río ML, López Menchero Oliva JC, García Cabezas MA (2016) [Use of topical
nitroglycerin in newborns with ischaemic injuries after vascular cannulation]. An
Pediatr (Barc) 85: 155-156. [Crossref]
12. Vasquez P, Burd A, Mehta R, Hiatt M, Hegyi T (2003) Resolution of peripheral artery catheter-induced ischemic injury following prolonged treatment with topical nitroglycerin ointment in a newborn: a case report. J Perinatol 23: 348-350. [Crossref]
13. Baserga MC, Puri A, Sola A (2002) The use of topical nitroglycerin ointment to treat peripheral tissue ischemia secondary to arterial line complications in neonates. J Perinatol 22: 416-419. [Crossref]
