Use of Urinary Bladder Matrix Conduits in a Rat Model of Sciatic Nerve Regeneration after Nerve Transection Injury
A B S T R A C T
Previous research has demonstrated the use of single-channel porcine-derived urinary bladder matrix (UBM) conduits in segmental-loss, peripheral nerve repairs as comparable to criterion-standard nerve autografts. This study aimed to replicate and expand upon this research with additional novel UBM conduits and coupled therapies. Fifty-four Wistar Albino rats were divided into 6 groups, and each underwent a surgical neurectomy to remove a 7-millimeter section of the sciatic nerve. Bridging of this nerve gap and treatment for each group was as follows: i) reverse autograft—the segmented nerve was reversed 180 degrees and used to reconnect the proximal and distal nerve stumps; ii) the nerve gap was bridged via a silicone conduit; iii) a single-channel UBM conduit; iv) a multi-channel UBM conduit; v) a single-channel UBM conduit identical to group 3 coupled with fortnightly transcutaneous electrical nerve stimulation (TENS); vi) or, a multi-channel UBM conduit identical to group 4 coupled with fortnightly TENS. The extent of nerve recovery was assessed by behavioural parameters: foot fault asymmetry scoring measured weekly for six weeks; electrophysiological parameters: compound muscle action potential (CMAP) amplitudes, measured at weeks 0 and 6; and morphological parameters: total fascicle areas, myelinated fiber counts, fiber densities, and fiber sizes measured at week 6. All the above parameters demonstrated recovery of the test groups (3-6) as being either comparable or less than that of reverse autograft, but none were shown to outperform reverse autograft. As such, UBM conduits may yet prove to be an effective treatment to repair relatively short segmental peripheral nerve injuries, but further research is required to demonstrate greater efficacy over nerve autografts.
Keywords
Porcine derived urinary bladder matrix, conduit, transcutaneous electrical nerve stimulation, sciatic nerve, peripheral nerve, regeneration, autograft, UBM, segmental defect, multi-channel nerve guide
Introduction
Injuries sustained to the peripheral nervous system (PNS) can be traumatic as the normal flow of information through sensory and motor neurons is disrupted. When damage occurs, peripheral nerves degenerate around the damaged site through Wallerian degeneration [1]. This process is slow and can lead to permanent loss of nerve function, while minor damage can lead to a temporary damage to the axons and Schwann cells [1, 2]. Ultimately, the damaged peripheral nerves regenerate only a fraction of lost motor and sensory function, and debridement of damaged nerves and cellular debris is often necessary for improved regeneration [3]. However, this form of repair is further complicated if the injury creates a nerve gap, making it difficult to achieve a tensionless primary neurorrhaphy [4]. If nothing is done to bridge this gap, new nerve fibers may grow disproportionately, and tissue is left without innervation [5]. In the case of trauma, scar tissue simply fills the space non-specifically. If space is preserved, neural tissue regrows more readily and fills in the gaps [6].
Nerve autografts have long been the standard treatment of segmental peripheral nerve gaps; however, this procedure is limited by the availability of expendable autologous nerves. As such, various conduits have been researched as potential alternatives to autografts [7-9].
Previous research showed that single-channel porcine-derived urinary bladder matrix (UBM) conduits were potentially effective in improving neuronal regeneration [10]. The aim of this study was to improve the recovery speed and quality of damaged peripheral nerves using multi-channel UBM conduits, both in the absence of and coupled with transcutaneous electrical nerve stimulation (TENS). Our hypothesis was that the use of multi-channel UBM conduits coupled with TENS therapy would result in a greater potential for neuronal regeneration.
Materials and Methods
I Nerve Guide Conduits
Single-channel and multi-channel UBM conduits were manufactured by ACell®, Inc. (Columbia, Maryland). HelixMark® silicone tubing (1.57 mm ID, 2.41 mm OD) was purchased from VWR (Part #62999-862) and cut to the proper length (10 mm) at ACell. The UBM and silicone conduits were packaged, sterilized by autoclave, and representative samples were tested for endotoxin using the limulus amebocyte lysate (LAL) method. The acceptable limit for designation of low endotoxin level or “pyrogen free” status was <1 endotoxin unit (EU)/mL. All samples were below the detection limit (0.1 EU/mL) for the endotoxin test.
II Animals
Adult female Wistar albino rats (Rattus norvegicus, n=54), weighing 250-300 g, and aged 15-36 weeks, were obtained from a breeding colony (Breeding Protocol 18-0302). Animals were caged in groups of three prior to surgery and separated into individual cages post-surgery. The rats were housed in a central animal care facility and provided with food and water ad libitum. All procedures were approved by the University’s Institutional Animal Care and Use Committee (IACUC Protocol 19-0401).
III Surgery
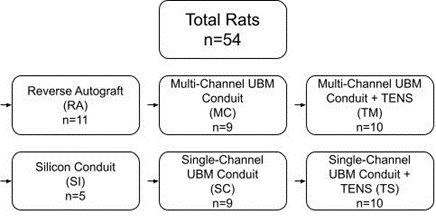
All rats underwent surgical transection of the sciatic nerve and were divided among six groups: i) Reverse Autograft (RA, n=11) as a positive control; ii) Silicone Conduit (SI, n=5) as a negative control; and four test groups: iii) Multi-Channel UBM Conduit (MC, n=9); iv) Single-Channel UBM Conduit (SC, n=9); v) Multi-Channel UBM Conduit coupled with TENS (TM, n=10); and vi) Single-Channel UBM Conduit coupled with TENS (TS, n=10). Figure 1 depicts the experimental design.
Figure 1: Experimental design.
Rats were anaesthetized via inhaled isoflurane (3%), and the left hindquarter was shaved and prepared aseptically. Prior to opening the surgical site, buprenorphine (1.0 mg/kg, Par Pharmaceutical, Irvine, CA) was injected subcutaneously, and bupivacaine (1.0-2.0 mg/kg, Lexicon Medical Supply, Tucson, AZ) mixed with lidocaine (1.0-2.0 mg/kg, Health Warehouse, Florence, KY) was injected intra-incisionally. A 3-cm incision was made at the left sciatic notch, parallel and posterior to the left femur. The biceps femoris was then reflected to expose the sciatic nerve. A 7-mm nerve gap was created in the midportion of the sciatic nerve with microsurgical scissors.
Nerve gaps were repaired with one of the following four procedures: i) the RA group had the transected portion of the nerve reversed 180 degrees and inserted as an autograft to bridge the gap between the proximal and distal neuronal stumps. Microsurgical anastomoses were performed using Covidien N-2547 8–0 sutures (eSutures, Mokena, IL). ii) For the SC and TS groups, a 10 mm (L) 1.5 mm (ID) single-channel UBM nerve guide conduit was used to bridge the sciatic nerve gap and secured using 8–0 nylon sutures. iii) For the MC and TM groups, a 10 mm (L) 1.5 mm (ID) multi-channel UBM nerve guide conduit was used to bridge the sciatic nerve gap and secured using 8-0 nylon sutures. The MC/TM and SC/TS conduits were briefly submerged in sterile water to make the conduits more pliable for insertion and easier to suture to the sciatic nerve. iv) For the SI group, a 10 mm (L) 1.57 mm (ID) silicone conduit was used to bridge the sciatic nerve gap and secured using 8–0 nylon sutures. All groups had the muscle and skin closed to cover the nerve using Covidien SN-5690 5–0 sutures (eSutures, Mokena, IL).
Immediately following the operation, triple antibiotic ointment (CVS Pharmacy, Woonsocket, RI) was applied to the incision site and bitter apple spray (Finish Line Pets, Tempe, AZ) was applied to the entire left hind limb to discourage autophagy. If rats developed pressure sores on the left foot or lower leg, the lesions were treated with Liquid Bandage (CVS Pharmacy, Woonsocket, RI) mixed with Metronidazole (generic, Petco) to discourage autophagy.
IV Pain and Distress Scoring
Postoperatively, each rat was monitored and assessed daily to minimize animal suffering using a scoring system for pain and distress developed and approved by the University’s IACUC (Table 1). The overall score was tabulated and used to help assess the status of each animal. A total score of 3 or less was considered normal. A total score of 4-6 indicated some evidence of pain or discomfort. A total score of 7-9 suggested ample evidence of suffering with some type of amelioration indicated. A total score of 10-12 was evidence of severe pain. Any single score of 3 (severe) for an independent variable automatically placed the animal in the 7-9 category. In accordance with the protocol approved by the University’s IACUC, a total score of six or higher, or any single score of 3 was reported to the University veterinarian and IACUC so the appropriate therapeutic measures including medication and dietary supplementation, could be initiated. Any animal that displayed severe signs of stress and pain, lost more than 20% of their healthy body weight, or presented with a severe infection around the treatment site was euthanized according to approved IACUC protocol.
Table 1: Pain and distress scoring criteria.
|
Body Weight 0 Normal 1
< 10% weight loss 2 10 - 15 % weight loss, eating 3 > 20% weight loss, not eating |
|
Appearance 0 Normal 1 Lack of grooming 2 Coat rough, possible nasal or ocular
discharge 3 Coat very rough, abnormal posture, eyes
sunken and glazed |
|
Clinical Signs 0 Normal 1 Diarrhea, constipation 2 Respiratory rates altered, respiratory
depth altered, skin tents 3 Cyanotic extremities, laboured breathing |
|
Unprovoked Behaviour 0 Normal 1 minor changes 2 Abnormal behaviours, less mobile, less
alert, inactive when activity expected 3 Paralysis, inability to remain upright,
shivering, convulsion |
V Foot Fault Asymmetry
Beginning the day after surgery, each rat underwent foot fault (FF) asymmetry testing. The method of testing and scoring used was the same as described by Nguyen et al. (2017) [10]. Testing was performed weekly for six weeks post-surgery. Each test was independently analysed by at least 2 blinded researchers to determine the rats’ FF score.
VI Electromyography
Each rat received transdermal electromyography to measure nerve conductivity. Electrode placement was the same as described by Wood et al. (2016) [11]. Compound muscle action potentials (CMAPs) were measured using a National Instruments PXI-1011 Chassis multi-function data acquisition instrument with LabVIEW software (National Instruments, Austin, TX).
Electromyography readings for each rat were assessed during surgery, immediately after opening the surgical site and prior to transection of the sciatic nerve to establish pre-procedure amplitudes and velocities and repeated at the end of the six-week period when the surgical site was reopened to harvest the sciatic nerve for histopathological staining prior to euthanasia.
VII Transcutaneous Electrical Nerve Stimulation
Rats in the TS and TM groups received Transcutaneous Electrical Nerve Stimulation (TENS) one week following surgery and every two weeks subsequent (weeks 1, 3, and 5) until the end point. Rats were anaesthetized through respiration of gaseous isoflurane at 2% and underwent a TENS session for 20 minutes. The TENS session treatment comprised placing the cathode of the TENS unit on the skin above the proximal end of the sciatic nerve transection, and the anode of the TENS unit on the skin near the distal end of the transection, and then stimulating the transection site by applying square 0.1 ms pulses (3V) at 20 Hz for the full 20 minutes.
VIII Histopathological Staining
Following euthanasia of each rat, the conduit and surrounding nerve was removed for analysis. Karnovsky’s fixative was used for nerve fixation and phosphate-buffered saline (PBS) was used for nerve preservation. Nerves were quartered and secondary fixation was performed with 1% osmium tetroxide. The nerves were dehydrated in acetone and embedded in Spurr’s resin. The excised nerve segments were cut 1µm thick beginning at the distal end. This provided the necessary area for analysis of growth and damage. Section images were obtained using a Nikon D3500 camera (Melville, NY) attached to a Zeiss Axiovert 135 microscope (Zeiss, Thornwood, NY). Analysis was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD).
A custom semi-automated process in ImageJ software, based on the procedure of Urso-Baiarda and Grobbelaar (2006), was used to calculate morphometric parameters for each nerve [11, 12]. Calculated morphometric parameters were total fascicle areas, myelinated fiber counts, fiber densities, fiber packing, and mean g-ratio values. A fiber comprises the axon and myelin together.
IX Statistical Analysis
Statistical significance for the electromyography data, comparing the pre- and post-surgery time points, was determined using a Mixed Model Analysis of Covariance with Post-hoc Tukey adjusted pairwise comparisons and a designated p-value of <0.05. Statistical significance for the Foot Fault Asymmetry testing was determined using a pairwise t-test with a pseudo-Bonferroni adjusted p-value of <0.001, comparing FF asymmetry scores between each group for every week.
Results
I Pain and Distress Scores
The mean weekly pain and distress scores for each group were below 3 for each time point (see Supplementary Table 1). Some pain is to be expected after surgery, and a score equal to or below 3 is considered normal (Figure 2) [13]. No statistical difference was observed between any group.
Figure 2: Mean weekly pain and distress scores for each group.
II Behavioural Evaluation of Sciatic Nerve Function
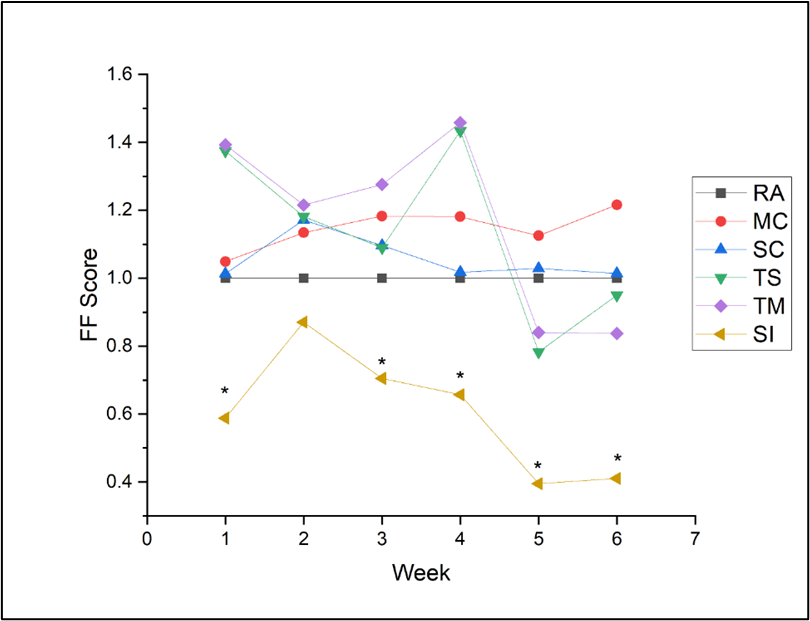
The mean FF score of each group for each week was normalized to the RA group so that the mean of the RA equalled 1.0, with a higher number indicating a greater level of recovery than the RA group and a lower number indicating a lower level of recovery. No significant difference in FF scores was found among rats repaired with reverse autograft and UBM conduit groups (i.e., RA, MC, SC, TM, and TC groups); however, two or more of these groups did outperform the silicone channel group (SI) each week, except for week 2 (Figure 3, also see Supplementary Table 2 for additional details of the statistical analysis).
III Electrophysiological Function of Injured Sciatic Nerve
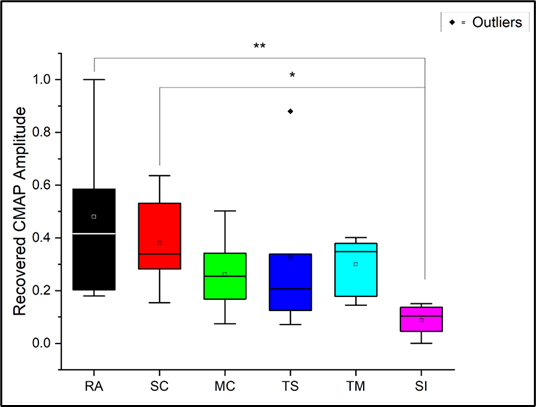
A recovery value for each rat was calculated by normalizing the mean difference of week 0 and week 6 CMAP amplitudes of each group to the RA group and ranging the values from 0 to 1. Rats repaired with reverse autograft and a single-channel UBM conduit without TENS (i.e., RA and SC groups) had a significantly greater recovery than the MC, TM, TC, and SI groups. A single outlier was found in the TS group. Excluding the outlier lowered the p-value for comparisons between both the RA and SC groups versus the SI group (see Supplementary Table 3); however, results with and without the outlier ultimately did not alter any statistically significant relationships (Figure 4).
IV Morphology
The healing of damaged nerves and the ability of Schwann cells to re-myelinate axons were assessed by histopathological analysis via light microscopy—an example of this may be seen in (Figure 5). The regenerated nerve fiber profiles were examined at six weeks post-injury. Table 2 compares the total fascicular area, total myelinated fiber counts, fiber densities, and mean fiber size of the TS and TM groups after six weeks of healing. The size of all nerve fibers for both groups ranged between 5.2 μm and 22.3 μm. No statistical difference was observed between these two groups. Control data from the RA or SI groups for the purpose of statistical comparison were not available for this portion of the analysis.
Figure 3: Weekly mean Foot Fault Scores normalized to RA group (1.0). *P<0.001, statistically significant when compared with SI group.
Figure 4: Recovery of CMAP amplitudes normalized to RA group and ranged 0-1.0. *P<0.05, statistically significant ** P<0.01, statistically significant.
Figure 5: Example of stained and sectioned nerve used for analysis. Leader arrow 1 points to a Schwann cell-derived myelin sheath, and leader arrow 2 points to the cross-section of an axonal fiber.
Discussion
Based on foot fault data and analysis of collected nerve samples, both single- and multi-channel UBM conduits were statistically similar to the results of reverse autograft testing. This remained true in the case of testing performed with and without TENS treatment following the procedure. All conduits analysed performed better than that of the silicone tubing utilized as a control. This conclusion is similar to that obtained by Nguyen et al. (2017) regarding RA compared to single-channel conduits at 6 weeks [10].
All rats in the study maintained low mean weekly pain and distress scores, indicating minimal pain associated with the procedures and implanted conduits. Maintenance of low pain and distress scores indicates the potential of this method as a treatment option as it demonstrates a safe procedure.
In examining the data collected from electromyography, the reverse autograft (acting as positive control) was the best option, followed by the single-channel conduits. Single-channel conduits produced a higher CMAP value than the other conduit options. By being able to recover a higher CMAP value, the single-channel conduits appear to have a better result in nerve healing than the other techniques and conduit designs utilized in this study. The single-channel conduits also produced a higher number of nerve fibers during the course of the study, which is encouraging for the regeneration of the damaged nerve.
The multi-channel conduits produced fewer fibers than the single-channel conduits, however the nerve fibers were thicker in the multi-channel conduits. Further studies would be useful in determining which of these two conduit designs would be more beneficial in the recovery process. From a potential nerve standpoint, having an increased fiber number would be assumed to be more beneficial as this would provide more fibers to transmit the electrical potential in the damaged nerve. Both of these conduits performed significantly and statistically better than the silicone conduits that were utilized as a negative control. However, the single-channel conduits proved easier and cheaper to produce and are recommended as the preferred conduit design. The single-channel conduits also were simpler to suture than the multi-channel conduits. Previous studies into nerve guidance conduits indicate that having more material present at the site can potentially interfere with the healing process, which would support the conclusion that single-channel conduits would be a better choice when utilizing a conduit [1].
Nerve autografts are commonly referred to as the “gold standard” of nerve repair following injury. However, even the ideal method of bridging is not without setbacks. One major issue faced with human nerve autografts is the length of the gap being filled. Current clinical research suggests that attempts to insert autografts into gaps greater than 3 centimeters tend to result in poor outcomes and improper healing [14]. This provides encouragement for the discovery of new techniques to bridge these gaps formed by nerve injury. Furthermore, autografts may produce undesirable symptoms in patients who undergo the procedure, including chronic pain and neuroma formation [15]. Despite these potential limitations, the autograft technique has been shown to produce the most promising and consistent healing and continues to remain at the forefront for nerve repair and regeneration.
While biologically derived conduits have limited clinical testing results in longer gaps, they prove to be readily available and can be adjusted to bridge the gap that is formed by the damaged nerve. These conduits may potentially also reduce the formation of scar tissue in the healing site and are formed of biologic material that encourages biocompatibility in the individual that requires them [16].
While the rats involved in the TENS portion of the study were found to have lower mean pain and distress scores than those involved in the other groups, there were no data suggesting that utilizing this technique encouraged nerve growth or healing and potentially may have interfered with the desired healing process. The lower pain and distress scores may indicate a possible method of increasing comfort in those who undergo the procedure and merits further research, along with using UBM conduits to bridge gaps longer than 3 centimeters in transected human nerves.
Conflicts of Interest
None.
Sources of Support
BYU and ACell.
Acknowledgement
A Cell provided the UBM conduits. Dr. Sandra Garrett, Dr. Dario Mizrachi, Dr. Scott Steffensen, Dr. Dennis Eggett, and Mike Standing provided valuable guidance. Undergraduates that assisted with animal care and surgeries were: Austin Thompson, Brandon Timmerman, Braxton Medrano, Brecken Campbell, Caleb Dixon, Cole Martin, Colton Sheperd, Corey Philpot, Douglas Ferry, Elijah Bingham, Elizabeth Mahoney, Gannon Jones, Gina Choi, Greggory Boatright II, Janelle Ting, Jared Tuttle, Jeremy Quackenbush, Joseph Turner, Kawika Dipko, Keaton Duty, Kirk Harter, Lincoln Kartchner, Madison Mehr, Marin Low, McKay Atkinson, Melinda Merrill, Mitchel Nelson, Morgan Skabelund, Parker Paulsen, Ryan DeCook, Samantha Layton, Sophia Schroeder, Tanner Thompson, and Zachary Thompson.
Author Contributions
Concept and design: JG, NS, BLR, ADC planned the studies; Experimental studies and data acquisition: JG, NS oversaw the animal work, BLR, ADC provided funding, equipment and lab space; Data analysis: JG, NS prepared tables, graphs, and figures, RLW analysed histology; Manuscript preparation: JG, NS, RLW, BLR, ADC wrote and edited the manuscript.
Funding
Funding for animal care and supplies was provided by Brigham Young University. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, nor in the decision to submit the paper for publication.
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham Young University (BYU Protocol 19-0401).
Biostatistics Statement
The statistical methods of this study were reviewed by the biostatistician of Brigham Young University in Provo, UT, USA, 84602.
Article Info
Article Type
Original ArticlePublication history
Received: Fri 11, Nov 2022Accepted: Wed 30, Nov 2022
Published: Wed 07, Dec 2022
Copyright
© 2023 Alonzo D. Cook. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2022.03.01
Author Info
Joel Goeckeritz Nathan Schank Ryan L Wood Beverly L Roeder Alonzo D. Cook
Corresponding Author
Alonzo D. CookNeuroscience Center, Brigham Young University, Provo, Utah, USA
Figures & Tables
Table 1: Pain and distress scoring criteria.
|
Body Weight 0 Normal 1
< 10% weight loss 2 10 - 15 % weight loss, eating 3 > 20% weight loss, not eating |
|
Appearance 0 Normal 1 Lack of grooming 2 Coat rough, possible nasal or ocular
discharge 3 Coat very rough, abnormal posture, eyes
sunken and glazed |
|
Clinical Signs 0 Normal 1 Diarrhea, constipation 2 Respiratory rates altered, respiratory
depth altered, skin tents 3 Cyanotic extremities, laboured breathing |
|
Unprovoked Behaviour 0 Normal 1 minor changes 2 Abnormal behaviours, less mobile, less
alert, inactive when activity expected 3 Paralysis, inability to remain upright,
shivering, convulsion |


References
1. Rotshenker S (2011)
Wallerian degeneration: the innate-immune response to traumatic nerve injury. J
Neuroinflammation 8: 109. [Crossref]
2. Richner M,
Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT et al. (2014) Peripheral nerve
injury modulates neurotrophin signaling in the peripheral and central nervous
system. Mol Neurobiol 50: 945-970. [Crossref]
3. Scholz T,
Krichevsky A, Sumarto A, Jaffurs D, Wirth G et al. (2009) Peripheral nerve
injuries: an international survey of current treatments and future
perspectives. J Reconstr Microsurg 25: 339-344. [Crossref]
4. Isaacs J, Browne T
(2014) Overcoming short gaps in peripheral nerve repair: conduits and human
acellular nerve allograft. Hand (N Y) 9: 131-137. [Crossref]
5. Bellamkonda RV
(2006) Peripheral nerve regeneration: an opinion on channels, scaffolds and
anisotropy. Biomaterials 27: 3515-3518. [Crossref]
6. Bozkurt A, Lassner
F, O'Dey D, Deumens R, Böcker A et al. (2012) The role of microstructured and
interconnected pore channels in a collagen-based nerve guide on axonal
regeneration in peripheral nerves. Biomaterials 33: 1363-1375. [Crossref]
7. Deal DN, Griffin
JW, Hogan MV (2012) Nerve conduits for nerve repair or reconstruction. J Am
Acad Orthop Surg 20: 63-68. [Crossref]
8. Griffin JW, Hogan
MV, Chhabra AB, Deal DN (2013) Peripheral nerve repair and reconstruction. J
Bone Joint Surg Am 95: 2144-2151. [Crossref]
9. Moore AM,
Kasukurthi R, Magill CK, Farhadi HF, Borschel GH et al. (2009) Limitations of
conduits in peripheral nerve repairs. Hand (N Y) 4: 180-186. [Crossref]
10. Nguyen L, Afshari
A, Kelm ND, Pollins AC, Shack RB et al. (2017) Bridging the Gap: Engineered
Porcine-derived Urinary Bladder Matrix Conduits as a Novel Scaffold for
Peripheral Nerve Regeneration. Ann Plast Surg 78: S328-S334. [Crossref]
11. Wood RL, Pitt WG,
Steffensen SC, Cook AD (2016) A comparison of lysophosphatidylcholine and crush
injury in a rat model of sciatic nerve regeneration. Adv Tissue Eng Regen
Med Open Access 1: 43-50.
12. Baiarda FU,
Grobbelaar AO (2006) Practical nerve morphometry. J Neurosci Methods
156: 333-341. [Crossref]
13. Bradshaw M,
Kartchner L, Boatright II G, Brown J, Campbell M et al. (2018) Realistic murine
model for streptozotocin-induced diabetic peripheral neuropathy. Int J Regen
Med 1: 1-10.
14. Hoben GM, Ee X,
Schellhardt L, Yan Y, Hunter DA et al. (2018) Increasing Nerve Autograft Length
Increases Senescence and Reduces Regeneration. Plast Reconstr Surg 142:
952-961. [Crossref]
15. Jain SA, Nydick J, Leversedge F, Power D, Styron J et al. (2021) Clinical Outcomes of Symptomatic Neuroma Resection and Reconstruction with Processed Nerve Allograft. Plast Reconstr Surg Glob Open 9: e3832. [Crossref]
16. Ray WZ, Mackinnon SE (2010) Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol 223: 77-85. [Crossref]
