Value of miR-30d and miR-146a as Prognostic Biomarkers for Heart Failure Development Post Myocardial Infarction
A B S T R A C T
Introduction: The development of heart failure (HF) following an acute myocardial infarction (AMI) is common and associated with poor clinical outcomes. In this context, the early identification of left ventricular remodelling that ultimately leads to HF remains challenging, with current biomarkers underperforming, and plasma microRNAs (miRs) have been proposed as functional biomarkers. Fibrotic and inflammatory processes are implicated in pathogenic remodelling, and miR-30d and miR-146a are reported to have regulatory function in these processes. This study aimed to determine if circulating levels of these miRs could be early predictors of HF development post myocardial infarction.
Method: We conducted a case-control study with 46 AMI patients who developed HF within 1 year (cases) matched with control AMI patients (1:2 ratio) who did not develop HF and measured plasma miRs via quantitative reverse transcription polymerase chain reaction.
Results: miR-30d was significantly upregulated in cases compared to controls (p<0.05), whereas miR-146a was not significantly different (p=0.57). ROC curve analysis for miR-30d demonstrated a modest sensitivity and specificity for this prediction (AUC=0.61, p<0.05). However, once adjusted for confounding factors such as atrial fibrillation and markers of inflammation, miR-30d was not found to be independently associated with HF development post myocardial infarction (OR 1.12 95% CI 0.99-1.27, p=0.08).
Conclusion: miR-30d and markers of inflammation were significantly elevated in patients who developed HF within 1 year of their AMI. Further research is needed to determine the regulatory role that miR-30d may play in HF and the utility it may have as a prognostic marker in this setting.
Keywords
Acute myocardial infarction, inflammation, microRNA, biomarker, heart failure
Introduction
The number of patients developing ischaemic heart failure (HF) following an acute myocardial infarction (AMI) is surging globally [1]. This has resulted in a significant impact on the healthcare system due to rates of rehospitalisation and the associated morbidity and mortality risk [2, 3]. Despite the latest techniques and advances in AMI treatment and management, the left ventricular (LV) remodelling process that ultimately leads to heart failure still presents a significant problem. Identification of patients at high risk of adverse LV remodelling is challenging, and current biomarkers of heart failure are limited in their capacity to predict early pathological remodelling that leads to the risk of heart failure [4]. There is a clear clinical need for novel biomarkers to classify patients according to their risk immediately after AMI.
Circulating microRNAs (miRNAs) have been gaining popularity over the last decade as promising prognostic biomarkers for a range of diseases, including cardiovascular disease. This is largely due to their stability in the circulation and the discovery that they can regulate almost 60% of the human protein coding genes [5]. The dysregulation of miRNA expression levels has been associated with many molecular pathways of atherosclerosis and arterial remodelling [6, 7]. Two candidates of interest are miR-30d and miR-146a. Both have been implicated in models of excessive inflammation and fibrosis, two processes that are tightly coupled to the pathogenesis of heart failure [8]. miR-30d has recently been proposed as an early biomarker for AMI and has been found to predict mortality in HF patients [9, 10]. miR-146a is upregulated in a range of inflammatory diseases and is associated with adverse LV remodelling parameters in AMI patients [11, 12]. The current study aimed to determine the predictive value of circulating miR-30d and miR-146a for identifying patients who developed new heart failure following an AMI.
Methods
I Study Population
We enrolled patients presenting to Wellington Hospital diagnosed with AMI and undergoing invasive management between January 2012 and October 2015. AMI was defined according to the third universal definition of myocardial infarction, where criteria for diagnosis include elevated troponin levels, symptoms suggestive of ischaemia for longer than 10 minutes, and/or ≥1 mm of new ST segment deviation or T wave inversion on an electrocardiogram in at least 2 contiguous leads [13].
Patients who were readmitted to the hospital with new-onset heart failure as the primary cause of admission within 12 months of their AMI were selected as the cases in this study cohort [14]. There were 46 cases identified, and these were then matched in a 1:2 ratio with patients who did not develop heart failure (controls) within the 12-month period following their AMI, bringing the total study cohort to 138 patients. Patients were matched based on their history of a prior AMI, age, gender, hypertension, and diabetes at the time of study enrolment. Patients were excluded from both the case and control cohort if they had a history of, or clinical signs of, heart failure at the time of enrolment. Participation was voluntary, and each patient gave informed written consent before enrolment. This study was reviewed and approved by the Upper A Regional Ethics Committee of the Health and Disabilities Ethics Committee of New Zealand (URA/11/05/2016).
II Data Collection
Patient demographics, past medical history, clinical risk characteristics and medications were collected prospectively by reviewing the medical records and the cardiac catheterization database. Patients with an eGFR of <45 mmol/L recorded at the time of study admission were classed as having renal dysfunction. Patients were defined as having atrial fibrillation (AF) if they presented with a known medical history of AF at the time of their AMI. Patient management and treatment decisions were at the discretion of the attending physician. Clinical follow-up was collected by patient contact via telephone at 1-year. Alongside this, patient hospital records were reviewed for supplemental data, and where necessary, a review of the case notes was performed, and the appropriate general practitioner was contacted to clarify the clinical outcomes further.
III Blood Collection
Blood samples were collected into tubes containing sodium citrate anti-coagulant prior to angiography either from the peripheral vein using a 21-gauge needle or from the arterial sheath immediately after insertion and prior to heparin administration. Plasma was separated by centrifugation at 1500 x g for 15 minutes and stored at -80°C until analysis.
IV Plasma miRNAs Determination
The plasma was centrifuged at 2000 x g for 10 mins and total RNA was extracted from 400 µL using the miRNeasy Serum/Plasma Kit (Qiagen). An exogenous spike-in synthetic miRNA, cel-miR-39-3p, was added to the plasma samples at 125 fmol/mL to correct for extraction efficiency. Reverse transcription of RNA was conducted using the Universal miRCURY LNATM cDNA Synthesis Kit (Qiagen), and the resulting cDNA was diluted 2-fold. Amplification was carried out using quantitative PCR with specific LNA primers to miR-30d-5p and miR-146a -5p and cel-miR-39-5p (Qiagen) using the Corbett Rotor-gene 6000 (Roche). Samples with Cq values over 35 were excluded from the final analysis. miR-30d and miR-146a levels were normalised to cel-miR-39 to obtain ΔCq values. Fold change values were obtained using the 2˄(-ΔΔCq) method, where ΔΔCq was calculated based on an average of control values or for matched cases and controls where indicated.
V Plasma Protein Determination
Plasma levels of IL-6, CRP, and NT-proBNP were determined using commercially available sandwich ELISAs according to the manufacturer’s instructions (IL-6 Uncoated ELISA Kit, Thermo Fisher Scientific, CRP Quantikine ELISA kit & NT-proBNP Duoset ELISA kits, both R&D systems). The assay sensitivity for the IL-6 ELISA was 2.0 pg/ml, CRP was 10 pg/ml, and NT-proBNP was 156 pg/ml.
VI Statistical Analyses
Normally distributed variables are given as means ± SEM and compared using a Student’s t-test. Variables with a skewed distribution are given as median with interquartile range and compared using the Mann-Whitney U-test. Categorical variables are described as frequencies and percentages of the cohort and compared using Chi-square or Fisher’s exact tests. Associations between the miRNAs and biochemical parameters were assessed with Spearman rank correlation coefficients. Univariate predictors of heart failure development were tested for independence using multinominal logistic regression analysis. A receiver operating characteristic (ROC) curve analysis was performed with miR-30d to assess the ability to discriminate between HF cases and controls. A difference of p<0.05 (two-sided) was considered to be statistically significant. All statistical analyses were conducted using SPSS v22 (IBM; New York, USA) or GraphPad Prism 7 (GraphPad Software Inc.).
For IL-6 levels, 11% patients had levels that fell below that of the lowest standard. As all these values were found to be above the blank measurements inside the assay, the method we employed to impute values for these patients in order to include them in the multivariate analysis was to analyse them using a linear regression fitted between the blank as 0 and standard 1. We chose not to apply this method to impute missing NT-proBNP values as less than one-third of the patients had detectable values in the range of the assay. Instead, we used a threshold where patients with values below 300 pg/ml where considered to have a low value of NT-proBNP, and those patients above 300 pg/ml were considered to have a high value of NT-proBNP. This value is the reported upper limit of normal by the European Society of Cardiology (ESC) guidelines in the acute setting to diagnose HF [15]. Therefore NT-proBNP as a categorical variable was included in the final multivariate model.
Results
I Baseline Characteristics
A total of 138 AMI patients were enrolled in the study, consisting of 46 cases and 92 controls. The baseline demographics and clinical characteristics are summarised in (Table 1). Atrial fibrillation was found at a significantly higher rate in cases compared with controls (Table 1). No other significant differences were identified in the remaining baseline demographic and clinical parameters.
II Plasma Level of miRNA and Relation to Heart Failure Development
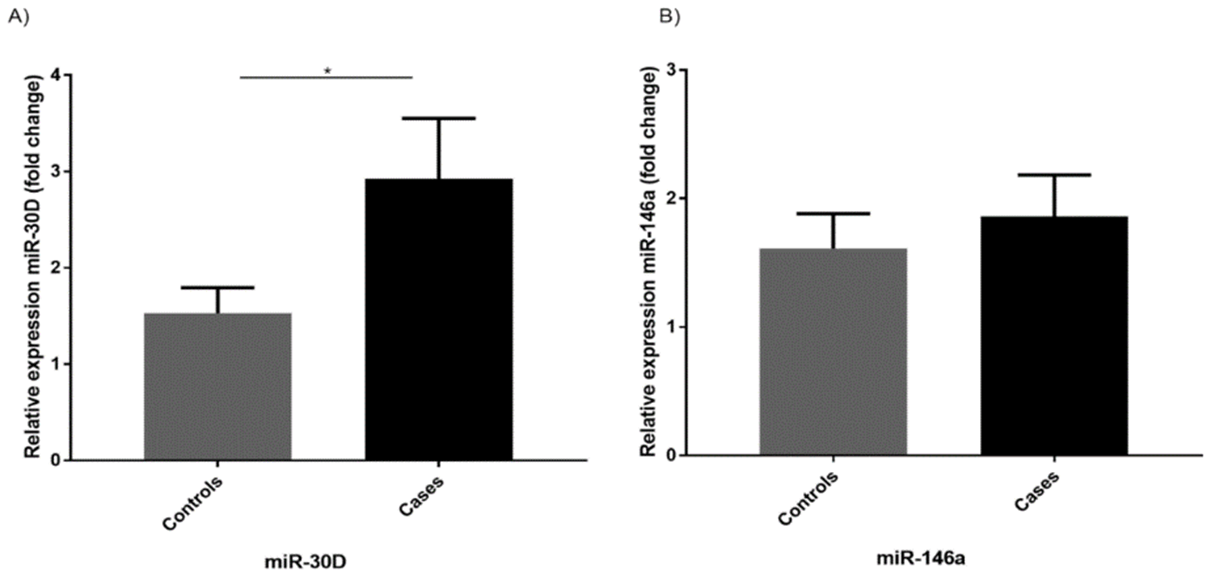
The average fold change of miR-30d was found to be significantly higher in patients who developed heart failure following their AMI (2.93 ± 0.62, p=0.02), whereas miR-146a was not found be significantly different in cases (1.86 ± 0.32, p=0.57) compared to controls (1.5 ± 0.26) (Figure 1). Ten patients from the control group were excluded from final qPCR analysis due to samples either failing to amplify or Cq values reaching above 35, indicating likely RNA degradation.
Table 1: Demographics of study population.
|
Demographics
|
Study Population n=138 |
Controls n=92 |
Cases n=46 |
P value |
|
Male, n (%) |
111 (80.4) |
74 (80.4) |
37 (80.4) |
1.0 |
|
Age, years (mean, SD) |
71.9 ± 9.74 |
71.2 ± 9.7 |
72.0 ± 9.9 |
0.94 |
|
BMI, kg/m2 (mean, SD) |
29.3 ± 5.77 |
28.8 ± 5.4 |
30.1 ± 6.4 |
0.25 |
|
Ethnicity, n (%) |
|
|
|
|
|
European |
121 (87.7) |
83 (90.2) |
38 (82.6) |
0.34 |
|
Maori & PI |
12 (8.7) |
7 (7.6) |
5 (10.9) |
|
|
Other |
5 (3.6) |
2 (2.2) |
3 (6.5) |
|
|
Risk Factors
|
|
|
|
|
|
Hypertension |
126 (91.3) |
84 (91.3) |
42 (91.3) |
1.0 |
|
Dyslipidaemia |
116 (84.1) |
78 (84.8) |
38 (82.6) |
0.74 |
|
Diabetes |
66 (47.8) |
41 (44.6) |
25 (54.3) |
0.28 |
|
Prior MI |
64 (46.4) |
42 (45.7) |
22 (47.8) |
0.81 |
|
Family Hx of CAD |
35 (25.4) |
26 (28.3) |
9 (19.6) |
0.27 |
|
Renal Dysfunction (eGFR<45) |
17 (12.3) |
9 (9.8) |
8 (17.4) |
0.20 |
|
Peripheral artery disease |
20 (14.5) |
12 (13) |
8 (17.4) |
0.50 |
|
Atrial Fibrillation |
17 (12.3) |
5 (5.4) |
12 (26.1) |
0.001 |
|
Stroke/TIA |
16 (11.6) |
9 (9.8) |
7 (15.2) |
0.35 |
|
Prior cardiac arrest |
3 (2.2) |
2 (2.2) |
1 (2.2) |
1.0 |
|
Smoking |
|
|
|
|
|
Current |
16 (11.6) |
9 (9.8) |
7 (15.2) |
0.64 |
|
Former |
68 (19.3) |
46 (50) |
22 (47.8) |
|
|
Never |
54 (39.1) |
37 (40.2) |
17 (37) |
|
|
Clinical Presentation
|
|
|
|
|
|
STEMI |
26 (18.8) |
19 (20.7) |
7 (15.2) |
0.44 |
|
NSTEMI |
112 (81.2) |
73 (79.3) |
39 (84.8) |
|
|
Clinical Management
|
|
|
|
|
|
CABG |
24 (17.4) |
15 (16.3) |
9 (19.6) |
0.48 |
|
PCI |
70 (50.7) |
50 (54.3) |
20 (43.5) |
|
|
Medical |
44 (31.9) |
27 (29.3) |
17 (37) |
|
|
GRACE Score
|
139 ± 28.7 |
140 ± 27.8 |
139 ± 30.7 |
0.96 |
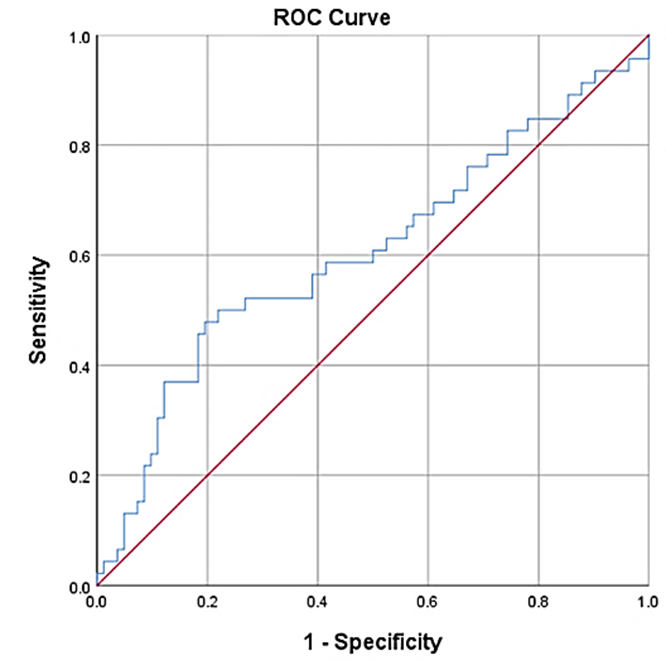
Further evaluation of miR-30d expression levels by ROC curve analysis demonstrated a moderate yet significant ability to predict those patients who were classified as cases (AUC=0.61, p<0.05); however, no specific cut-point for expression levels of these miRNAs could be identified conclusively (Figure 2).
Figure 1: Level of miR-30d and miR-146a in study population. A) Level of miR-30d was significantly higher in those patients that developed heart failure (cases) vs. those that did not develop heart failure (controls). B) Level of miR-146a were not significantly higher in cases vs controls. Significant differences were detected by Students t-test, *p<0.05. Data shown for 46 cases and 82 controls, presented as mean ± SEM.
Figure 2: The receiver-operator characteristic curves for miR-30d in the study population. miR-30d gave an area under the curve of 0.61, 95% CI of 0.5-0.71, p=0.046.
III Biochemical Parameters and Relation to Heart Failure Development
Inflammation was assessed by measurement of circulating CRP and IL-6. CRP was found to be significantly elevated in cases compared to controls [3.82 (1.81-16.3) vs. 1.97 (0.92-5.19) mg/L respectively], (Table 2). IL-6 was found to be significantly higher in cases compared to controls [(3.50 (2.11-6.68) vs 2.14 (1.23-4.0) pg/ml)], (Table 2). Levels of NT-proBNP was detectable in 35 (25%) patients at the time of study admission. There was no statistically significant difference detected between cases and controls (Table 2) in NT-proBNP levels, nor any significant differences in the proportion of patients with elevated levels (data not shown).
Table 2: Biochemical parameters of study population.
|
Biochemical markers |
Study Population |
Controls |
Cases |
P value |
|
IL-6 (pg/ml) n=138 |
2.55 (1.38-4.90) |
2.14 (1.23-4.00) |
3.50 (2.11-6.68) |
0.002 |
|
CRP (mg/L) n=138 |
2.48 (1.30-8.39) |
1.97 (0.92-5.19) |
3.82 (1.81-16.3) |
0.019 |
|
NT-proBNP (pg/ml) n=35 |
664 (309-1267) |
541 (309-1242) |
896 (322-1535) |
0.23 |
IV Relationship between Circulating miRNA and Biochemical Parameters
Table 3 summarises the level of correlation found between levels of the miRNAs, the inflammatory markers and NT-proBNP. miR-30d and miR-146a were highly correlated with each other (rho=0.59, p<0.0001), along with CRP and IL-6 (rho=0.6, p<0.0001). MiR-30d and CRP were weakly correlated with each other (rho=0.18, p<0.05).
Table 3: Correlation matrix of biochemical and miRNA parameters.
|
|
|
miR-30d |
miR-146a |
IL-6 |
CRP |
NT-proBNP |
|
miR-30d |
Correlation Coefficient |
- |
0.59 |
0.04 |
0.18 |
0.12 |
|
|
P value |
- |
<0.0001 |
0.68 |
0.04 |
0.19 |
|
miR-146a |
Correlation Coefficient |
0.59 |
- |
-0.09 |
0.08 |
0.04 |
|
|
P value |
<0.0001 |
- |
0.30 |
0.35 |
0.69 |
|
IL-6 |
Correlation Coefficient |
0.04 |
-0.09 |
- |
0.60 |
0.28 |
|
|
P value |
0.65 |
0.30 |
- |
<0.0001 |
0.001 |
|
CRP |
Correlation Coefficient |
0.18 |
0.08 |
0.60 |
- |
0.15 |
|
|
P value |
0.04 |
0.35 |
<0.0001 |
- |
0.08 |
|
NT-proBNP |
Correlation Coefficient |
0.12 |
0.04 |
0.28 |
0.15 |
- |
|
|
P value |
0.19 |
0.69 |
0.001 |
0.08 |
- |
V Levels of miRNA as a Prognostic Indicator for Heart Failure Development Following AMI
In the univariate analysis, miR-30d was significantly elevated in the cases, but we observed no difference in miR-146a between cases and controls (Figure 1). The inflammatory markers IL-6 and CRP were also elevated in the cases versus the controls (Table 2). We included these variables into the multivariate logistic regression model, along with atrial fibrillation and NT-proBNP as a binary variable (elevated or not) to test for independence. Those with 300 pg/ml NT-proBNP or above were considered to have elevated levels (19% of patients), and those below 300 pg/ml were considered to have low levels (81% of patients).
Those patients with missing miR-30d and/or miR-146a values were excluded from the model rather than imputing values for these parameters, as there was no robust model for imputation (n=10). Atrial fibrillation was the only variable that remained an independent predictor for heart failure development following AMI in this cohort (OR 8.01, 95%CI 2.35-27.8), although we observed an odds ratio of 1.12 (95% CI 0.99-1.27) for miR-30d with a p value of 0.08 (Table 4).
Table 4: Multivariate logistic regression model.
|
Variable |
Odds Ratio |
95% Confidence Interval |
P value |
|
Atrial Fibrillation |
8.09 |
2.35-27.8 |
0.001 |
|
miR-30d |
1.12 |
0.99-1.27 |
0.08 |
|
IL-6 |
1.04 |
0.98-1.10 |
0.18 |
|
CRP |
1.01 |
0.99-1.03 |
0.24 |
|
Nt-proBNP |
0.59 |
0.20-1.77 |
0.35 |
Discussion
The aim of this study was to identify whether circulating levels of miR-30d and miR-146a were associated with HF development in patients following their AMI. The results show that miR-30d, but not miR-146a, was significantly elevated in patients who developed new-onset HF within one year of their AMI. miR-30d was found to be a modest predictor of HF development on the basis of ROC curve analysis; however, despite a statistical trend in the multivariate model, miR-30d was not found to be independently associated with HF. NT-proBNP is the gold standard biomarker in HF and is routinely used in the diagnosis and to guide therapy in HF patients [15]. However, NT-proBNP performs poorly as a prognostic biomarker in the setting of HF post-AMI, as it is only detectable after significant changes in LV wall stress have occurred [4, 16]. There remains a clear clinical need to risk stratify patients following their acute MI to identify those patients who might benefit from a more intensive management approach, including greater use of imaging technologies, novel drugs and closer follow-up.
miR-30d has been found to outperform cardiac troponin I in early diagnosis of AMI, with levels found to peak at 4 hours post symptom onset [9]. It is thought to be released by cardiomyocytes in response to myocardial wall stress, and the miR-30 family are circulating markers of interest for the diagnosis of myocardial hypertrophy and fibrosis [17-19]. These results are favourable when hypothesising whether miR-30d may be an important candidate as an early biomarker of risk following myocardial damage. There is a growing body of evidence to support the ability of several miRNAs to predict outcome in acute HF patients [20]. In particular, miR-30d has not only been associated with response to cardiac resynchronization therapy but is also reported to predict 1-year mortality in HF patients [10, 17]. In this study, we demonstrate that miR-30d is significantly elevated in those AMI patients who go on to develop HF within 1-year of their event. ROC curve analysis suggests a modest sensitivity and specificity for this prediction, and despite an odds ratio of 1.12, miR-30d was not found to be independently associated with HF after adjustment for atrial fibrillation and markers of inflammation, due to the 95% confidence interval spanning from 0.99-1.27.
Although it is possible that a larger cohort may establish an independent relationship due to a greater statistical power, our current results suggest that the prognostic value of miR-30d is modest in the setting of post-infarction HF. Several studies have demonstrated cell-to-cell transport of miRNAs, and their potential as paracrine signalling molecules, supporting the fact that miRNAs are not merely by-products of myocardial necrosis and are likely to have precise functions. Definitive proof of the functional role of miR-30d needs to be elucidated to further understand its potential role as a biomarker in this field.
We observed a modest correlation between miR-30d and CRP levels. Inflammation is regarded as a central mechanism to LV remodelling, which ultimately leads to the development of HF [21]. Our study shows that both CRP and IL-6 levels were significantly increased in patients who developed HF, indicating that they experienced higher levels of inflammation; however, they did not remain as independent predictors after adjustment for other variables. This finding is not overly surprising given that CRP is a broad marker of inflammation, and although numerous studies have shown an association between CRP and adverse outcomes following an MI, the predictive power is not sufficient to be a recommended measurement in current guidelines [22]. Often inflammatory cytokines are the preferred measure by study investigators due to their more direct relationship to atherosclerosis, but currently, there is no consensus on the best cytokine measure [23, 24]. IL-6 induces the hepatic synthesis of CRP, and levels of IL-6 have been associated with decreased cardiac function following MI [25, 26]. It is recently reported that miR-30d levels were associated with the IL-6 signalling pathway in a model of LV remodelling, and whilst our markers of inflammation and miR-30d levels did not retain independence as predictors of heart failure, it remains likely that the role of inflammation and miRNAs are important to the pathogenesis of HF development [27].
Limitations
Due to the historic nature of our study, coupled with the relatively modest case numbers, subgroup analysis based on the ejection fraction could not be performed on our cohort. Similarly, analysis based on troponin levels was not possible due to the tertiary nature of Wellington Hospital as the troponin assays used differed between referring hospitals. Together this limits our ability to interpret these results in the setting of LV remodelling; however, this study chose to focus on the presentation of new HF, which is the clinical endpoint of adverse LV remodelling. Furthermore, it is possible that in a larger cohort, a significant relationship between miR-30d and HF development would be found in multivariate analysis.
We sampled miR-30d and miR-146a during the acute phase of the MI at an opportunistic timepoint prior to angiography. We have not established that this is the optimal time for measuring these miRNAs, or the time course of release in the acute phase following an MI. Additionally, circulating miRNAs may be differentially expressed in the plasma and serum fractions of the same individual. Therefore, caution must be taken when comparing miRNAs reported from various studies, where different methodologies are employed to generate patterns of miRNA expression in patients [28].
Conclusion
In this matched case-control study, we have identified that levels of miR-30d and markers of inflammation were significantly elevated in patients who went on to develop HF within 1 year of their AMI. However, once adjusted for confounding factors such as atrial fibrillation, these markers were not independently predictive of this outcome. Despite this, it is likely miR-30d has value in the setting of post-infarction HF, and further research is needed to elucidate its role in the regulatory process associated with HF.
Funding
This study was supported by funding from Research for Life (Wellington Medical Research Foundation).
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 08, Dec 2020Accepted: Sat 19, Dec 2020
Published: Wed 31, Mar 2021
Copyright
© 2023 Ana S. Holley. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2021.01.01
Author Info
Ana S. Holley Kirsty M. Danielson Scott A. Harding Peter D. Larsen
Corresponding Author
Ana S. HolleyWellington Cardiovascular Research Group, Wellington, New Zealand
Figures & Tables
Table 1: Demographics of study population.
|
Demographics
|
Study Population n=138 |
Controls n=92 |
Cases n=46 |
P value |
|
Male, n (%) |
111 (80.4) |
74 (80.4) |
37 (80.4) |
1.0 |
|
Age, years (mean, SD) |
71.9 ± 9.74 |
71.2 ± 9.7 |
72.0 ± 9.9 |
0.94 |
|
BMI, kg/m2 (mean, SD) |
29.3 ± 5.77 |
28.8 ± 5.4 |
30.1 ± 6.4 |
0.25 |
|
Ethnicity, n (%) |
|
|
|
|
|
European |
121 (87.7) |
83 (90.2) |
38 (82.6) |
0.34 |
|
Maori & PI |
12 (8.7) |
7 (7.6) |
5 (10.9) |
|
|
Other |
5 (3.6) |
2 (2.2) |
3 (6.5) |
|
|
Risk Factors
|
|
|
|
|
|
Hypertension |
126 (91.3) |
84 (91.3) |
42 (91.3) |
1.0 |
|
Dyslipidaemia |
116 (84.1) |
78 (84.8) |
38 (82.6) |
0.74 |
|
Diabetes |
66 (47.8) |
41 (44.6) |
25 (54.3) |
0.28 |
|
Prior MI |
64 (46.4) |
42 (45.7) |
22 (47.8) |
0.81 |
|
Family Hx of CAD |
35 (25.4) |
26 (28.3) |
9 (19.6) |
0.27 |
|
Renal Dysfunction (eGFR<45) |
17 (12.3) |
9 (9.8) |
8 (17.4) |
0.20 |
|
Peripheral artery disease |
20 (14.5) |
12 (13) |
8 (17.4) |
0.50 |
|
Atrial Fibrillation |
17 (12.3) |
5 (5.4) |
12 (26.1) |
0.001 |
|
Stroke/TIA |
16 (11.6) |
9 (9.8) |
7 (15.2) |
0.35 |
|
Prior cardiac arrest |
3 (2.2) |
2 (2.2) |
1 (2.2) |
1.0 |
|
Smoking |
|
|
|
|
|
Current |
16 (11.6) |
9 (9.8) |
7 (15.2) |
0.64 |
|
Former |
68 (19.3) |
46 (50) |
22 (47.8) |
|
|
Never |
54 (39.1) |
37 (40.2) |
17 (37) |
|
|
Clinical Presentation
|
|
|
|
|
|
STEMI |
26 (18.8) |
19 (20.7) |
7 (15.2) |
0.44 |
|
NSTEMI |
112 (81.2) |
73 (79.3) |
39 (84.8) |
|
|
Clinical Management
|
|
|
|
|
|
CABG |
24 (17.4) |
15 (16.3) |
9 (19.6) |
0.48 |
|
PCI |
70 (50.7) |
50 (54.3) |
20 (43.5) |
|
|
Medical |
44 (31.9) |
27 (29.3) |
17 (37) |
|
|
GRACE Score
|
139 ± 28.7 |
140 ± 27.8 |
139 ± 30.7 |
0.96 |
Table 2: Biochemical parameters of study population.
|
Biochemical markers |
Study Population |
Controls |
Cases |
P value |
|
IL-6 (pg/ml) n=138 |
2.55 (1.38-4.90) |
2.14 (1.23-4.00) |
3.50 (2.11-6.68) |
0.002 |
|
CRP (mg/L) n=138 |
2.48 (1.30-8.39) |
1.97 (0.92-5.19) |
3.82 (1.81-16.3) |
0.019 |
|
NT-proBNP (pg/ml) n=35 |
664 (309-1267) |
541 (309-1242) |
896 (322-1535) |
0.23 |
Table 3: Correlation matrix of biochemical and miRNA parameters.
|
|
|
miR-30d |
miR-146a |
IL-6 |
CRP |
NT-proBNP |
|
miR-30d |
Correlation Coefficient |
- |
0.59 |
0.04 |
0.18 |
0.12 |
|
|
P value |
- |
<0.0001 |
0.68 |
0.04 |
0.19 |
|
miR-146a |
Correlation Coefficient |
0.59 |
- |
-0.09 |
0.08 |
0.04 |
|
|
P value |
<0.0001 |
- |
0.30 |
0.35 |
0.69 |
|
IL-6 |
Correlation Coefficient |
0.04 |
-0.09 |
- |
0.60 |
0.28 |
|
|
P value |
0.65 |
0.30 |
- |
<0.0001 |
0.001 |
|
CRP |
Correlation Coefficient |
0.18 |
0.08 |
0.60 |
- |
0.15 |
|
|
P value |
0.04 |
0.35 |
<0.0001 |
- |
0.08 |
|
NT-proBNP |
Correlation Coefficient |
0.12 |
0.04 |
0.28 |
0.15 |
- |
|
|
P value |
0.19 |
0.69 |
0.001 |
0.08 |
- |
Table 4: Multivariate logistic regression model.
|
Variable |
Odds Ratio |
95% Confidence Interval |
P value |
|
Atrial Fibrillation |
8.09 |
2.35-27.8 |
0.001 |
|
miR-30d |
1.12 |
0.99-1.27 |
0.08 |
|
IL-6 |
1.04 |
0.98-1.10 |
0.18 |
|
CRP |
1.01 |
0.99-1.03 |
0.24 |
|
Nt-proBNP |
0.59 |
0.20-1.77 |
0.35 |
References
1. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC et al. (2009) Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol 53: 13-20. [Crossref]
2. Braunschweig F, Cowie MR, Auricchio A (2011) What are the costs of heart failure? Europace 13: ii13-ii17. [Crossref]
3. Givertz MM, Teerlink JR, Albert NM, Canary CAW, Collins SP et al. (2013) Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail 19: 371-389. [Crossref]
4. Talwar S, Squire IB, Downie PF, Mccullough AM, Campton MC et al. (2000) Profile of plasma N-terminal proBNP following acute myocardial infarction; correlation with left ventricular systolic dysfunction. Eur Heart J 21: 1514-1521. [Crossref]
5. Bajan S, Hutvagner G (2014) Regulation of miRNA processing and miRNA mediated gene repression in cancer. Microrna 3: 10-17. [Crossref]
6. Urbich C, Kuehbacher A, Dimmeler S (2008) Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79: 581-588. [Crossref]
7. Papageorgiou N, Tousoulis D, Androulakis E, Siasos G, Briasoulis A et al. (2012) The role of microRNAs in cardiovascular disease. Curr Med Chem 19: 2605-2610. [Crossref]
8. Van Linthout S, Tschöpe C (2017) Inflammation - Cause or Consequence of Heart Failure or Both? Curr Heart Fail Rep 14: 251-265. [Crossref]
9. Jia K, Shi P, Han X, Chen T, Tang H et al. (2016) Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol Med Rep 14: 184-194. [Crossref]
10. Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y et al. (2017) Circulating miR-30d Predicts Survival in Patients with Acute Heart Failure. Cell Physiol Biochem 41: 865-874. [Crossref]
11. Scalbert E, Bril A (2008) Implication of microRNAs in the cardiovascular system. Curr Opin Pharmacol 8: 181-188. [Crossref]
12. Liu X, Dong Y, Chen S, Zhang G, Zhang M et al. (2015) Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 132: 233-241. [Crossref]
13. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR et al. (2012) Third universal definition of myocardial infarction. Circulation 126: 2020-2035. [Crossref]
14. Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS et al. (2018) National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart Lung Circ 27: 1123-1208. [Crossref]
15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18: 891-975. [Crossref]
16. Bjorklund E, Jernberg T, Johanson P, Venge P, Dellborg M et al. (2006) Admission N-terminal pro-brain natriuretic peptide and its interaction with admission troponin T and ST segment resolution for early risk stratification in ST elevation myocardial infarction. Heart 92: 735-740. [Crossref]
17. Melman YF, Shah R, Danielson K, Xiao J, Simonson B et al. (2015) Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 131: 2202-2216. [Crossref]
18. Pan W, Zhong Y, Cheng C, Liu B, Wang L et al. (2013) MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 8: e53950. [Crossref]
19. Fang L, Ellims AH, Moore X, White DA, Taylor AJ et al. (2015) Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med 13: 314. [Crossref]
20. Siasos G, Bletsa E, Stampouloglou PK, Oikonomou E, Tsigkou V et al. (2020) MicroRNAs in cardiovascular disease. Hellenic J Cardiol 61: 165-173. [Crossref]
21. Burchfield JS, Xie M, Hill JA (2013) Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation 128: 388-400. [Crossref]
22. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M et al. (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37: 267-315. [Crossref]
23. Moriya J (2019) Critical roles of inflammation in atherosclerosis. J Cardiol 73: 22-27. [Crossref]
24. Kristono GA, Holley AS, Lakshman P, Brunton O'Sullivan MM, Hardin SA et al. (2020) Association between inflammatory cytokines and long-term adverse outcomes in acute coronary syndromes: A systematic review. Heliyon 6: e03704. [Crossref]
25. Groot HE, Al Ali L, van der Horst ICC, Schurer RAJ, van der Werf HW et al. (2019) Plasma interleukin 6 levels are associated with cardiac function after ST-elevation myocardial infarction. Clin Res Cardiol 108: 612-621. [Crossref]
26. Ritschel VN, Seljeflot I, Arnesen H, Halvorsen S, Weiss T et al. (2014) IL-6 signalling in patients with acute ST-elevation myocardial infarction. Results Immunol 4: 8-13. [Crossref]
27. Danielson KM, Shah R, Yeri A, Liu X, Garcia FC et al. (2018) Plasma Circulating Extracellular RNAs in Left Ventricular Remodeling Post-Myocardial Infarction. EBioMedicine 32: 172-181. [Crossref]
28. Mompeón A, Ortega Paz L, Vidal Gómez X, Costa TJ, Pérez Cremades D et al. (2020) Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci Rep 10: 5373. [Crossref]
